Appropriateness of Cotrimoxazole Prophylactic Therapy Among HIV/AIDS Patients in Public Hospitals in Eastern Ethiopia: A Retrospective Evaluation of Clinical Practice
- PMID: 30042677
- PMCID: PMC6048359
- DOI: 10.3389/fphar.2018.00727
Appropriateness of Cotrimoxazole Prophylactic Therapy Among HIV/AIDS Patients in Public Hospitals in Eastern Ethiopia: A Retrospective Evaluation of Clinical Practice
Abstract
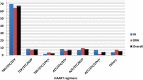
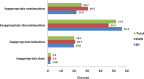
Background: Cotrimoxazole prophylactic therapy (CPT) is a feasible, cost-effective, and safe way of using cotrimoxazole intervention to reduce HIV/AIDS related morbidities and mortalities associated with opportunistic infections. Despite its effectiveness in reducing the incidence of opportunistic infections, the actual drug utilization process has been shown to deviate from World Health Organization (WHO) guideline in Ethiopia. This study, therefore, aims to evaluate CPT among HIV/AIDS patients in Jugel Hospital (JH), Harar and Dilchora Referral Hospital (DRH), Dire Dawa, Eastern Ethiopia. Methods: A cross sectional study was conducted to evaluate the use of cotrimoxazole as prophylactic therapy. In this study, 556 medical records (305 in JH and 251 in DRH) of HIV/AIDS patients who had been taking CPT within September 2015-August 2016 were reviewed. Systematic random sampling was employed to obtain medical records from the sampling frame. Data were abstracted from the patient medical records using structured checklist customized from the WHO guideline. The data were entered into Epi-data 3.1 and exported to and analyzed with statistical Package for Social Sciences (SPSS) version 20. The finding was evaluated against the WHO guideline on the use of cotrimoxazole prophylaxis in HIV/AIDS patients. Descriptive statistics was used to present the data in tables, figures and pie chart. Results: Majority of the HIV/AIDS patients who had been taking CPT were adults (95.9%), female (61.2%), married (43.7%), Orthodox Christian (54.3%), and attended primary school (40.1%). At the initiation of CPT, most of the patients were at WHO clinical stage III (40.8%). The major comorbid illnesses identified were tuberculosis and pneumocystis-jiroveci pneumonia. Initially, majority of the patients were at CD4 count of less than 350 cells/mm3 (n = 504, 90.6%). Greater proportion of patients started CPT prior to initiating antiretroviral therapy (ART). Most of the patients took CPT for greater than 6 months. The primary reasons for premature discontinuation of CPT were CD4 greater than 350 cells/mm3, severe sulfa allergy and first trimester of pregnancy. Generally, the use of cotrimoxazole prophylaxis was consistent with the WHO guideline for indication to start (n = 519, 93.3%) and dose (n = 552, 99.28%), despite the presence of contraindication in 6.65% patients. Conclusion: In reference to the WHO guideline, the use of CPT was found to be fully appropriate in nearly two-thirds of HIV/AIDS patients. For the rest patients, inappropriate use of cotrimoxazole was observed based on the WHO criteria for initiation, discontinuation, continuation and dose with rate of discontinuation being the dominant one. Such practice may lead to adverse health outcomes including adverse drug reactions and negative treatment outcome.
Keywords: Eastern Ethiopia; HIV/AIDS; cotrimoxazole; drug use evaluation; prophylaxis.
Figures

Similar articles
-
Evaluation of cotrimoxazole use as a preventive therapy among patients living with HIV/AIDS in Gondar University Referral Hospital, northwestern Ethiopia: a retrospective cross-sectional study.HIV AIDS (Auckl). 2016 Jul 7;8:125-33. doi: 10.2147/HIV.S103081. eCollection 2016. HIV AIDS (Auckl). 2016. PMID: 27462178 Free PMC article.
-
Pattern of and reasons for antiretroviral therapy regimen change among adult HIV/AIDS patients at regional hospital in Eastern Ethiopia: A 10-year retrospective study.SAGE Open Med. 2019 Feb 1;7:2050312119827092. doi: 10.1177/2050312119827092. eCollection 2019. SAGE Open Med. 2019. PMID: 30746143 Free PMC article.
-
Retrospective evaluation of cotrimoxazole use as preventive therapy in people living with HIV/AIDS in Boru Meda Hospital.BMC Pharmacol Toxicol. 2014 Feb 8;15:4. doi: 10.1186/2050-6511-15-4. BMC Pharmacol Toxicol. 2014. PMID: 24507658 Free PMC article.
-
Discontinuing Pneumocystis jirovecii Pneumonia Prophylaxis in HIV-Infected Patients With a CD4 Cell Count <200 cells/mm3.Ann Pharmacother. 2015 Dec;49(12):1343-8. doi: 10.1177/1060028015605113. Epub 2015 Sep 10. Ann Pharmacother. 2015. PMID: 26358129 Review.
-
The Efficacy of Cotrimoxazole for the Prevention of Pneumocystis jirovecii Pneumonia Among HIV-Exposed and Infected Children: A Systematic Review.Epidemiologia (Basel). 2025 Feb 13;6(1):8. doi: 10.3390/epidemiologia6010008. Epidemiologia (Basel). 2025. PMID: 39982260 Free PMC article. Review.
Cited by
-
Drug hypersensitivity in HIV infection.Curr Opin Allergy Clin Immunol. 2019 Aug;19(4):272-282. doi: 10.1097/ACI.0000000000000545. Curr Opin Allergy Clin Immunol. 2019. PMID: 31145192 Free PMC article. Review.
-
Incidence of common opportunistic infections among HIV-infected children on ART at Debre Markos referral hospital, Northwest Ethiopia: a retrospective cohort study.BMC Infect Dis. 2020 Jan 16;20(1):50. doi: 10.1186/s12879-020-4772-y. BMC Infect Dis. 2020. PMID: 31948393 Free PMC article.
-
Half-life time prediction of developing first-line antiretroviral treatment failure and its risk factors among TB and HIV co-infected children in Northwest Ethiopia; multi setting historical follow-up study.BMC Pediatr. 2022 Mar 3;22(1):114. doi: 10.1186/s12887-022-03177-6. BMC Pediatr. 2022. PMID: 35241036 Free PMC article.
-
Predictive factors of viral load high-risk events for virological failure in HIV/AIDS patients receiving long-term antiviral therapy.BMC Infect Dis. 2021 May 18;21(1):448. doi: 10.1186/s12879-021-06162-z. BMC Infect Dis. 2021. PMID: 34006230 Free PMC article.
-
Incidence of Allergic Drug Eruption due to Cotrimoxazole in HIV-Positive Individuals with CD4 ≤200 Cells/ul.J Int Assoc Provid AIDS Care. 2023 Jan-Dec;22:23259582221146946. doi: 10.1177/23259582221146946. J Int Assoc Provid AIDS Care. 2023. PMID: 36700255 Free PMC article.
References
-
- Alemayehu M., Yisehak Y., Alaro W., Alemayehu B. (2017). Opportunistic infections among HIV/AIDS patients taking ante-retroviral therapy at tertiary care hospital in Wolaita zone, southern Ethiopia. J. AIDS Clin. Res. 8:2 10.4172/2155-6113.1000665 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

