The Interplay of Notch Signaling and STAT3 in TLR-Activated Human Primary Monocytes
- PMID: 30042932
- PMCID: PMC6048282
- DOI: 10.3389/fcimb.2018.00241
The Interplay of Notch Signaling and STAT3 in TLR-Activated Human Primary Monocytes
Abstract
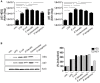
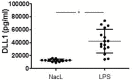
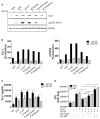
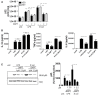
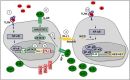
The highly conserved Notch signaling pathway essentially participates in immunity through regulation of developmental processes and immune cell activity. In the adaptive immune system, the impact of the Notch cascade in T and B differentiation is well studied. In contrast, the function, and regulation of Notch signaling in the myeloid lineage during infection is poorly understood. Here we show that TLR signaling, triggered through LPS stimulation or in vitro infection with various Gram-negative and -positive bacteria, stimulates Notch receptor ligand Delta-like 1 (DLL1) expression and Notch signaling in human blood-derived monocytes. TLR activation induces DLL1 indirectly, through stimulated cytokine expression and autocrine cytokine receptor-mediated signal transducer and activator of transcription 3 (STAT3). Furthermore, we reveal a positive feedback loop between Notch signaling and Janus kinase (JAK)/STAT3 pathway during in vitro infection that involves Notch-boosted IL-6. Inhibition of Notch signaling by γ-secretase inhibitor DAPT impairs TLR4-stimulated accumulation of NF-κB subunits p65 in the nucleus and subsequently reduces LPS- and infection-mediated IL-6 production. The reduced IL-6 release correlates with a diminished STAT3 phosphorylation and reduced expression of STAT3-dependent target gene programmed death-ligand 1 (PD-L1). Corroborating recombinant soluble DLL1 and Notch activator oxaliplatin stimulate STAT3 phosphorylation and expression of immune-suppressive PD-L1. Therefore we propose a bidirectional interaction between Notch signaling and STAT3 that stabilizes activation of the transcription factor and supports STAT3-dependent remodeling of myeloid cells toward an immuno-suppressive phenotype. In summary, the study provides new insights into the complex network of Notch regulation in myeloid cells during in vitro infection. Moreover, it points to a participation of Notch in stabilizing TLR-mediated STAT3 activation and STAT3-mediated modulation of myeloid functional phenotype through induction of immune-suppressive PD-L1.
Keywords: DLL1; Notch signaling; PD-L1; STAT3; TLR; infection.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

