Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial
- PMID: 30043039
- PMCID: PMC6233839
- DOI: 10.1001/jamaophthalmol.2018.3255
Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial
Erratum in
-
Errors in eTable 6 in the Supplement.JAMA Ophthalmol. 2019 Apr 1;137(4):467. doi: 10.1001/jamaophthalmol.2019.0032. JAMA Ophthalmol. 2019. PMID: 30816939 Free PMC article. No abstract available.
Abstract
Importance: Ranibizumab is a viable treatment option for eyes with proliferative diabetic retinopathy (PDR) through 2 years. However, longer-term results are needed.
Objective: To evaluate efficacy and safety of 0.5-mg intravitreous ranibizumab vs panretinal photocoagulation (PRP) over 5 years for PDR.
Design, setting, and participants: Diabetic Retinopathy Clinical Research Network multicenter randomized clinical trial evaluated 394 study eyes with PDR enrolled February through December 2012. Analysis began in January 2018.
Interventions: Eyes were randomly assigned to receive intravitreous ranibizumab (n = 191) or PRP (n = 203). Frequency of ranibizumab was based on a protocol-specified retreatment algorithm. Diabetic macular edema could be managed with ranibizumab in either group.
Main outcomes and measures: Mean change in visual acuity (intention-to-treat analysis) was the main outcome. Secondary outcomes included peripheral visual field loss, development of vision-impairing diabetic macular edema, and ocular and systemic safety.
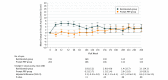
Results: The 5-year visit was completed by 184 of 277 participants (66% excluding deaths). Of 305 enrolled participants, the mean (SD) age was 52 (12) years, 135 (44%) were women, and 160 (52%) were white. For the ranibizumab and PRP groups, the mean (SD) number of injections over 5 years was 19.2 (10.9) and 5.4 (7.9), respectively; the mean (SD) change in visual acuity letter score was 3.1 (14.3) and 3.0 (10.5) letters, respectively (adjusted difference, 0.6; 95% CI, -2.3 to 3.5; P = .68); the mean visual acuity was 20/25 (approximate Snellen equivalent) in both groups at 5 years. The mean (SD) change in cumulative visual field total point score was -330 (645) vs -527 (635) dB in the ranibizumab (n = 41) and PRP (n = 38) groups, respectively (adjusted difference, 208 dB; 95% CI, 9-408). Vision-impairing diabetic macular edema developed in 27 and 53 eyes in the ranibizumab and PRP groups, respectively (cumulative probabilities: 22% vs 38%; hazard ratio, 0.4; 95% CI, 0.3-0.7). No statistically significant differences between groups in major systemic adverse event rates were identified.
Conclusions and relevance: Although loss to follow-up was relatively high, visual acuity in most study eyes that completed follow-up was very good at 5 years and was similar in both groups. Severe vision loss or serious PDR complications were uncommon with PRP or ranibizumab; however, the ranibizumab group had lower rates of developing vision-impairing diabetic macular edema and less visual field loss. Patient-specific factors, including anticipated visit compliance, cost, and frequency of visits, should be considered when choosing treatment for patients with PDR. These findings support either anti-vascular endothelial growth factor therapy or PRP as viable treatments for patients with PDR.
Trial registration: ClinicalTrials.gov Identifier: NCT01489189.
Conflict of interest statement
Figures

Comment in
-
Incorrect Data in Supplement.JAMA Ophthalmol. 2019 Apr 1;137(4):467. doi: 10.1001/jamaophthalmol.2019.0001. JAMA Ophthalmol. 2019. PMID: 30816936 No abstract available.
References
-
- National diabetes fact sheet, 2007. Centers for Disease Control and Prevention. https://stacks.cdc.gov/view/cdc/5613/cdc_5613_DS1.pdf. Accessed July 5, 2018.
-
- The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy: Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88(7):583-600. - PubMed
-
- Sivaprasad S, Prevost AT, Vasconcelos JC, et al. ; CLARITY Study Group . Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193-2203. doi:10.1016/S0140-6736(17)31193-5 - DOI - PubMed
-
- Gross JG, Glassman AR, Jampol LM, et al. ; Writing Committee for the Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi:10.1001/jama.2015.15217 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

