Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions
- PMID: 30043395
- PMCID: PMC6492113
- DOI: 10.1111/nph.15344
Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions
Abstract
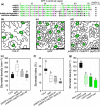
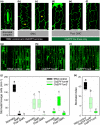
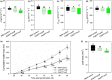
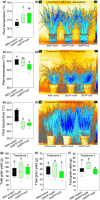
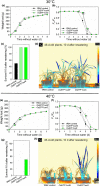
Much of humanity relies on rice (Oryza sativa) as a food source, but cultivation is water intensive and the crop is vulnerable to drought and high temperatures. Under climate change, periods of reduced water availability and high temperature are expected to become more frequent, leading to detrimental effects on rice yields. We engineered the high-yielding rice cultivar 'IR64' to produce fewer stomata by manipulating the level of a developmental signal. We overexpressed the rice epidermal patterning factor OsEPF1, creating plants with substantially reduced stomatal density and correspondingly low stomatal conductance. Low stomatal density rice lines were more able to conserve water, using c. 60% of the normal amount between weeks 4 and 5 post germination. When grown at elevated atmospheric CO2 , rice plants with low stomatal density were able to maintain their stomatal conductance and survive drought and high temperature (40°C) for longer than control plants. Low stomatal density rice gave equivalent or even improved yields, despite a reduced rate of photosynthesis in some conditions. Rice plants with fewer stomata are drought tolerant and more conservative in their water use, and they should perform better in the future when climate change is expected to threaten food security.
Keywords: climate change; drought; epidermal pattering factor; heat stress; rice; stomata; water conservation.
© 2018 The Authors. New Phytologist © 2018 New Phytologist Trust.
Figures

References
-
- Ainsworth EA. 2008. Rice production in a changing climate: a meta‐analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biology 14: 1642–1650.
-
- Bouman B. 2009. How much water does rice use? Rice today 8: 28–29.
-
- van Campen JC, Yaapar MN, Narawatthana S, Lehmeier C, Wanchana S, Thakur V, Chater C, Kelly S, Rolfe SA, Quick WP et al 2016. Combined chlorophyll fluorescence and transcriptomic analysis identifies the P3/P4 transition as a key stage in rice leaf photosynthetic development. Plant Physiology 170: 1655–1674. - PMC - PubMed
-
- Casson S, Gray JE. 2008. Influence of environmental factors on stomatal development. New Phytologist 178: 9–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

