Activation of ERBB4 in Glioblastoma Can Contribute to Increased Tumorigenicity and Influence Therapeutic Response
- PMID: 30044378
- PMCID: PMC6116191
- DOI: 10.3390/cancers10080243
Activation of ERBB4 in Glioblastoma Can Contribute to Increased Tumorigenicity and Influence Therapeutic Response
Abstract
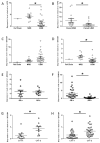
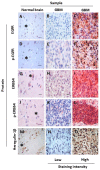
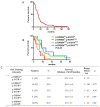
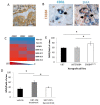
Glioblastoma (GBM) is often resistant to conventional and targeted therapeutics. ErbB2 Receptor Tyrosine Kinase 4 (ERBB4) is expressed throughout normal brain and is an oncogene in several pediatric brain cancers; therefore, we investigated ERBB4 as a prognostic marker and therapeutic target in GBM. Using RT-qPCR, we quantified mRNA encoding total ERBB4 and known ERBB4 variants in GBM and non-neoplastic normal brain (NNB) samples. Using immunohistochemistry, we characterized the localization of total and phosphorylated ERBB4 (p-ERBB4) and EGFR protein in archived GBM samples and assessed their association with patient survival. Furthermore, we evaluated the effect of ERBB4 phosphorylation on angiogenesis and tumorigenicity in GBM xenograft models. Total ERBB4 mRNA was significantly lower in GBM than NNB samples, with the juxtamembrane JM-a and cytoplasmic CYT-2 variants predominating. ERBB4 protein was ubiquitously expressed in GBM but was not associated with patient survival. However, high p-ERBB4 in 11% of archived GBM samples, independent of p-EGFR, was associated with shorter patient survival (12.0 ± 3.2 months) than was no p-ERBB4 (22.5 ± 9.5 months). Increased ERBB4 activation was also associated with increased proliferation, angiogenesis, tumorigenicity and reduced sensitivity to anti-EGFR treatment in xenograft models. Despite low ERBB4 mRNA in GBM, the functional effects of increased ERBB4 activation identify ERBB4 as a potential prognostic and therapeutic target.
Keywords: EGFR; ERBB4; GBM; prognosis; therapy.
Conflict of interest statement
Panitumumab was supplied to TGJ by Amgen, and dacomitinib was supplied to TGJ and NGG by Pfizer through a grant-in-aid.
Figures


References
-
- Inda M.M., Bonavia R., Mukasa A., Narita Y., Sah D.W., Vandenberg S., Brennan C., Johns T.G., Bachoo R., Hadwiger P., et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24:1731–1745. doi: 10.1101/gad.1890510. - DOI - PMC - PubMed
-
- Van den Bent M.J., Brandes A.A., Rampling R., Kouwenhoven M.C., Kros J.M., Carpentier A.F., Clement P.M., Frenay M., Campone M., Baurain J.F., et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. - DOI - PMC - PubMed
-
- Brown P.D., Krishnan S., Sarkaria J.N., Wu W., Jaeckle K.A., Uhm J.H., Geoffroy F.J., Arusell R., Kitange G., Jenkins R.B., et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North central cancer treatment group study N0177. J. Clin. Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

