Comparative efficacy and safety of licensed treatments for previously treated non-small cell lung cancer: A systematic review and network meta-analysis
- PMID: 30044785
- PMCID: PMC6059384
- DOI: 10.1371/journal.pone.0199575
Comparative efficacy and safety of licensed treatments for previously treated non-small cell lung cancer: A systematic review and network meta-analysis
Abstract
Purpose: This systematic review with network meta-analysis compared the efficacy and safety of currently licensed second-line treatments in patients with late stage non-small cell lung cancer (NSCLC).
Methods: Randomised controlled trials (RCTs) of participants with advanced/metastatic NSCLC receiving second/third line treatments were screened. We searched electronic databases (MEDLINE; EMBASE; Web of Science) from January, 2000 to July, 2017. Two reviewers screened bibliographic records, extracted data, and assessed risk of bias of included studies. The outcomes were overall survival (OS), progression-free survival (PFS), and drug-related grade 3-5 adverse-events (AEs). We pooled study-specific hazard ratios (HR; for OS and PFS) and risk ratios (RR; for AEs) using conventional and network-meta-analyses, and ranked interventions by the surface under the cumulative ranking curve.
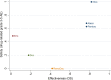
Findings: We included 11 RCTs (7,581 participants) comparing nine drugs. All drugs except for erlotinib significantly improved OS compared to docetaxel. Nivolumab was the highest ranking drug followed by atezolizumab and pembrolizumab. There was no significant difference in OS across these three drugs (HR = 0.98, 95% CI 0.79, 1.21 for nivolumab vs atezolizumab; HR = 0.98, 95% CI 0.77, 1.25 for nivolumab vs pembrolizumab). For PFS, ramucirumab + docetaxel and nivolumab were the drugs with the highest ranking. All interventions except ramucirumab + docetaxel had a reduced risk for severe drug-related AEs vs. docetaxel. Of the drugs with the highest ranking on AEs, nivolumab was significantly safer compared to atezolizumab (RR = 0.55, 95% CI 0.38, 0.79) or pembrolizumab (RR = 0.52, 95% CI 0.34, 0.81).
Implications: Nivolumab, pembrolizumab and atezolizumab exhibited superior benefit/risk balance compared to other licensed drugs used late stage NSCLC. Our results indicate that the use of immunotherapies in people diagnosed with non-specific late stage NSCLC should be promoted. The use of docetaxel may now be judged irrelevant as a comparator intervention for approval of new drugs for second line treatment of NSCLC.
Study registration number: PROSPERO CRD42017065928.
Conflict of interest statement
XA, AT, MC, PR, GJMT, AC: none to declare. PJS reports grants from Bristol Myers Squibb, Roche, and MSD outside the submitted work. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology: official journal of the European Society for Medical Oncology. 2016;27(suppl 5):v1–v27. Epub 2016/09/25. 10.1093/annonc/mdw326 . - DOI - PubMed
-
- Royal College of Physicians. National Lung Cancer Audit. 2015.
-
- Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N. A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer. Health Technol Assess. 2001;5(32):1–195. Epub 2002/06/18. . - PubMed
-
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4:1 Epub 2015/01/03. 10.1186/2046-4053-4-1 ; PubMed Central PMCID: PMCPMC4320440. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

