Treatment for HIV-associated cryptococcal meningitis
- PMID: 30045416
- PMCID: PMC6513250
- DOI: 10.1002/14651858.CD005647.pub3
Treatment for HIV-associated cryptococcal meningitis
Abstract
Background: Cryptococcal meningitis is a severe fungal infection that occurs primarily in the setting of advanced immunodeficiency and remains a major cause of HIV-related deaths worldwide. The best induction therapy to reduce mortality from HIV-associated cryptococcal meningitis is unclear, particularly in resource-limited settings where management of drug-related toxicities associated with more potent antifungal drugs is a challenge.
Objectives: To evaluate the best induction therapy to reduce mortality from HIV-associated cryptococcal meningitis; to compare side effect profiles of different therapies.
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL, MEDLINE (PubMed), Embase (Ovid), LILACS (BIREME), African Index Medicus, and Index Medicus for the South-East Asia Region (IMSEAR) from 1 January 1980 to 9 July 2018. We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), ClinicalTrials.gov, and the ISRCTN registry; and abstracts of select conferences published between 1 July 2014 and 9 July 2018.
Selection criteria: We included randomized controlled trials that compared antifungal induction therapies used for the first episode of HIV-associated cryptococcal meningitis. Comparisons could include different individual or combination therapies, or the same antifungal therapies with differing durations of induction (less than two weeks or two or more weeks, the latter being the current standard of care). We included data regardless of age, geographical region, or drug dosage. We specified no language restriction.
Data collection and analysis: Two review authors independently screened titles and abstracts identified by the search strategy. We obtained the full texts of potentially eligible studies to assess eligibility and extracted data using standardized forms. The main outcomes included mortality at 2 weeks, 10 weeks, and 6 months; mean rate of cerebrospinal fluid fungal clearance in the first two weeks of treatment; and Division of AIDS (DAIDS) grade three or four laboratory events. Using random-effects models we determined pooled risk ratio (RR) and 95% confidence interval (CI) for dichotomous outcomes and mean differences (MD) and 95% CI for continuous outcomes. For the direct comparison of 10-week mortality, we assessed the certainty of the evidence using the GRADE approach. We performed a network meta-analysis using multivariate meta-regression. We modelled treatment differences (RR and 95% CI) and determined treatment rankings for two-week and 10-week mortality outcomes using surface under the cumulative ranking curve (SUCRA). We assessed transitivity by comparing distribution of effect modifiers between studies, local inconsistency through a node-splitting approach, and global inconsistency using design-by-treatment interaction modelling. For the network meta-analysis, we applied a modified GRADE approach for assessing the certainty of the evidence for 10-week mortality.
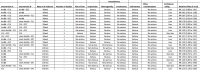
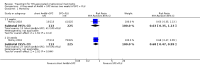
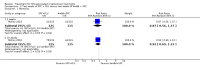
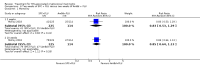
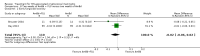
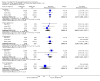
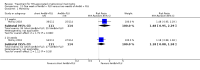
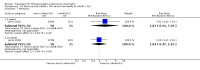
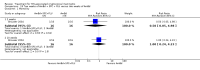
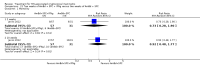
Main results: We included 13 eligible studies that enrolled 2426 participants and compared 21 interventions. All studies were carried out in adults, and all but two studies were conducted in resource-limited settings, including 11 of 12 studies with 10-week mortality data.In the direct pairwise comparisons evaluating 10-week mortality, one study from four sub-Saharan African countries contributed data to several key comparisons. At 10 weeks these data showed that those on the regimen of one-week amphotericin B deoxycholate (AmBd) and flucytosine (5FC) followed by fluconazole (FLU) on days 8 to 14 had lower mortality when compared to (i) two weeks of AmBd and 5FC (RR 0.62, 95% CI 0.42 to 0.93; 228 participants, 1 study), (ii) two weeks of AmBd and FLU (RR 0.58, 95% CI 0.39 to 0.86; 227 participants, 1 study), (iii) one week of AmBd with two weeks of FLU (RR 0.49, 95% CI 0.34 to 0.72; 224 participants, 1 study), and (iv) two weeks of 5FC and FLU (RR 0.68, 95% CI 0.47 to 0.99; 338 participants, 1 study). The evidence for each of these comparisons was of moderate certainty. For other outcomes, this shortened one-week AmBd and 5FC regimen had similar fungal clearance (MD 0.05 log10 CFU/mL/day, 95% CI -0.02 to 0.12; 186 participants, 1 study) as well as lower risk of grade three or four anaemia (RR 0.31, 95% CI 0.16 to 0.60; 228 participants, 1 study) compared to the two-week regimen of AmBd and 5FC.For 10-week mortality, the comparison of two weeks of 5FC and FLU with two weeks of AmBd and 5FC (RR 0.92, 95% CI 0.69 to 1.23; 340 participants, 1 study) or two weeks of AmBd and FLU (RR 0.85, 95% CI 0.64 to 1.13; 339 participants, 1 study) did not show a difference in mortality, with moderate-certainty evidence for both comparisons.When two weeks of combination AmBd and 5FC was compared with AmBd alone, pooled data showed lower mortality at 10 weeks (RR 0.66, 95% CI 0.46 to 0.95; 231 participants, 2 studies, moderate-certainty evidence).When two weeks of AmBd and FLU was compared to AmBd alone, there was no difference in 10-week mortality in pooled data (RR 0.94, 95% CI 0.55 to 1.62; 371 participants, 3 studies, low-certainty evidence).One week of AmBd and 5FC followed by FLU on days 8 to 14 was the best induction therapy regimen after comparison with 11 other regimens for 10-week mortality in the network meta-analysis, with an overall SUCRA ranking of 88%.
Authors' conclusions: In resource-limited settings, one-week AmBd- and 5FC-based therapy is probably superior to other regimens for treatment of HIV-associated cryptococcal meningitis. An all-oral regimen of two weeks 5FC and FLU may be an alternative in settings where AmBd is unavailable or intravenous therapy cannot be safely administered. We found no mortality benefit of combination two weeks AmBd and FLU compared to AmBd alone. Given the absence of data from studies in children, and limited data from high-income countries, our findings provide limited guidance for treatment in these patients and settings.
Conflict of interest statement
Mark W Tenforde declares no relevant conflicts of interest. Adrienne E Shapiro has received salary compensation from the University of Washington, as a trainee postdoctoral fellow in Infectious Diseases. A portion of the salary derives from an NIH T32 training grant. Benjamin Rouse declares no relevant conflicts of interest. Joseph N Jarvis has been an investigator in several clinical trials of therapies for HIV‐associated cryptococcal meningitis. He has previously received grant support through his institution from Gilead Sciences Europe (investigator initiated award for the Ambition Phase II Trial) Tianjing Li declares no relevant conflicts of interest. Ingrid Eshun‐Wilson declares no relevant conflicts of interest. Nathan Ford declares no relevant conflicts of interest.
Figures

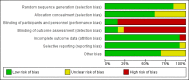


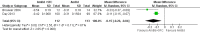



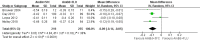


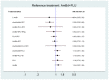
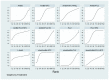

















































Update of
-
Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings.Cochrane Database Syst Rev. 2008 Oct 8;(4):CD005647. doi: 10.1002/14651858.CD005647.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2018 Jul 25;7:CD005647. doi: 10.1002/14651858.CD005647.pub3. PMID: 18843697 Updated.
References
References to studies included in this review
Beardsley 2016 {published data only}
Brouwer 2004 {published data only}
-
- Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, et al. Combination antifungal therapies for HIV‐associated cryptococcal meningitis: a randomised trial. Lancet 2004;363(9423):1764‐7. - PubMed
Day 2013 {published data only}
Jackson 2012 {published data only}
Jarvis 2012 {published data only}
Jarvis 2018 {published data only}
-
- Jarvis JN, Leeme TB, Molefi M, Chofle AA, Bidwell G, Tsholo K, et al. Short course high‐dose liposomal amphotericin B for HIV‐associated cryptococcal meningitis: a phase‐II randomised controlled trial. Clinical Infectious Diseases 2018 June 26 [Epub ahead of print]. [DOI: 10.1093/cid/ciy515] - DOI - PMC - PubMed
Leenders 1997 {published data only}
-
- Leenders AC, Reiss P, Portegies P, Clezy K, Hop WC, Hoy J, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS‐associated cryptococcal meningitis. AIDS 1997;11(12):1463‐71. - PubMed
Loyse 2012 {published data only}
Mayanja‐Kizza 1998 {published data only}
-
- Mayanja‐Kizza H, Oishi K, Mitarai S, Yamashita H, Nalongo K, Watanabe K, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clinical Infectious Diseases 1998;26(6):1362‐6. - PubMed
Molloy 2018 {published data only}
-
- Molloy S, Kanyama C, Heyderman R, Loyse A, Kouanfack C, Chanda D, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. New England Journal of Medicine 2018;378(11):1004‐17. - PubMed
Nussbaum 2010 {published data only}
-
- Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, et al. Combination flucytosine and high‐dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. Clinical Infectious Diseases 2010;50(3):338‐44. - PMC - PubMed
Pappas 2009 {published data only}
-
- Pappas PG, Chetchotisakd P, Larsen RA, Manosuthi W, Morris MI, Anekthananon T. A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV‐associated cryptococcal meningitis. Clinical Infectious Diseases 2009;48(12):1775‐83. - PubMed
van der Horst 1997 {published data only}
-
- Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. New England Journal of Medicine 1997;337(1):15‐21. - PubMed
References to studies excluded from this review
Bicanic 2008 {published data only}
-
- Bicanic T, Wood R, Meintjes G, Rebe K, Brouwer A, Loyse A, et al. High‐dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV‐infected patients: a randomized trial. Clinical Infectious Diseases 2008;47(1):123‐30. - PubMed
Bisson 2013 {published data only}
-
- Bisson GP, Molefi M, Bellamy S, Thakur R, Steenhoff A, Tamuhla N, et al. Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clinical Infectious Diseases 2013;56(8):1165‐73. - PubMed
Boulware 2014b {published data only}
Bozzette 1991 {published data only}
-
- Bozzette SA, Larsen RA, Chiu J, Leal MAE, Jacobsen J, Rothman P, et al. A placebo‐controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. New England Journal of Medicine 1991;324(9):580‐4. - PubMed
Brouwer 2007 {published data only}
Chariyalertsak 2002 {published data only}
-
- Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clinical Infectious Diseases 2002;32(2):277‐84. - PubMed
Chetchotisakd 2004 {published data only}
-
- Chetchotisakd P, Sungkanuparph S, Thinkhamrop B, Mootsikapun P, Boonyaprawit P. A multicentre, randomized, double‐blind, placebo‐controlled trial of primary cryptococcal meningitis prophylaxis in HIV‐infected patients with severe immune deficiency. HIV Medicine 2004;5(3):140‐3. - PubMed
Chotmongkol 1997 {published data only}
-
- Chotmongkol V, Sukeepaisarncharoen W, Thavornpitak Y. Comparison of amphotericin B, flucytosine and itraconazole with amphotericin B and flucytosine in the treatment of cryptococcal meningitis in AIDS. Journal of the Medical Association of Thailand 1997;80(7):416‐25. - PubMed
Chotmongkol 2005 {published data only}
-
- Chotmongkol V, Arayawichanont A, Sawanyawisuth K, Thavornpitak Y. Initial treatment of cryptococcal meningitis in AIDS. Southeast Asian Journal of Tropical Medicine and Public Health 2005;36(1):170‐3. - PubMed
de Gans 1992 {published data only}
-
- Gans J, Portegies P, Tiessens G, Schattenkerk JKME, Boxtel CJ, Ketel RJ, et al. Itraconazole compared with amphotericin B plus flucytosine in AIDS patients with cryptococcal meningitis. AIDS 1992;6(2):185‐90. - PubMed
Hamill 2010 {published data only}
-
- Hamill RJ, Sobel JD, El‐Sadr W, Johnson PC, Graybill JR, Javaly K, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS‐associated acute cryptococcal meningitis: a randomized, double‐blind clinical trial of efficacy and safety. Clinical Infectious Diseases 2010;51(2):225‐32. - PubMed
Jadhav 2010 {published data only}
-
- Jadhav MP, Bamba A, Shinde VM, Gogtay N, Kshirsagar NA, Bichile LS, et al. Liposomal amphotericin B (Fungisome) for the treatment of cryptococcal meningitis in HIV/AIDS patients in India: a multicentric, randomized controlled trial. Journal of Postgraduate Medicine 2010;56(2):71‐5. - PubMed
Joly 1996a {published data only}
-
- Joly V, Aubry P, Ndayiragide A, Carriere I, Kawa E, Mlika‐Cabanne N, et al. Randomized comparison of amphotericin B deoxycholate dissolved in dextrose or Intralipid for the treatment of AIDS‐associated cryptococcal meningitis. Clinical Infectious Diseases 1996;23(3):556‐62. - PubMed
Joly 1996b {published data only}
-
- Joly V, Geoffray C, Reynes J, Goujard C, Méchali D, Maslo C, et al. Amphotericin B in a lipid emulsion for the treatment of cryptococcal meningitis in AIDS patients. Journal of Antimicrobial Chemotherapy 1996;38(1):117‐26. - PubMed
Larsen 1990 {published data only}
-
- Larsen RA, Leal MAE, Chan LS. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Annals of Internal Medicine 1990;113(3):183‐7. - PubMed
Makadzange 2010 {published data only}
-
- Makadzange AT, Ndhlovu CE, Takarinda K, Reid M, Kurangwa M, Gona P, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub‐saharan Africa. Clinical Infectious Diseases 2010;50(11):1532‐8. - PubMed
Mfinanga 2015 {published data only}
-
- Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community‐based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open‐label, randomised controlled trial. Lancet 2015;385(9983):2173‐82. - PubMed
Mootsikapun 2003 {published data only}
-
- Mootsikapun P, Chetchotisakd P, Anunnatsiri S, Choksawadphinyo K. The efficacy of fluconazole 600 mg/day versus itraconazole 600 mg/day as consolidation therapy of cryptococcal meningitis in AIDS patients. Journal of the Medical Association of Thailand 2003;86(4):293‐8. - PubMed
Newton 2002 {published data only}
-
- Newton PN, Thai le H, Tip NQ, Short JM, Chierakul W, Rajanuwong A, et al. A randomized, double‐blind, placebo‐controlled trial of acetazolamide for the treatment of elevated intracranial pressure in cryptococcal meningitis. Clinical Infectious Diseases 2002;36(5):769‐72. - PubMed
Pappas 2004 {published data only}
-
- Pappas PG, Bustamante B, Ticona E, Hamill RJ, Johnson PC, Reboli A. Recombinant interferon‐gamma 1b as adjunctive therapy for AIDS‐related acute cryptococcal meningitis. Journal of Infectious Diseases 2004;189(12):2185‐91. - PubMed
Powderly 1992 {published data only}
-
- Powderly WG, Saag MS, Cloud GA, Robinson P, Meyer RD, Jacobson JM, et al. A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. The NIAID AIDS Clinical Trials Group and Mycoses Study Group. New England Journal of Medicine 1992;326(12):783‐8. - PubMed
Powderly 1995 {published data only}
-
- Powderly WG, Finkelstein D, Feinberg J, Frame P, He W, Horst C, et al. A randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. New England Journal of Medicine 1995;332(11):700‐5. - PubMed
Rhein 2016 {published data only}
Saag 1991 {published data only}
-
- Saag MS, Powderly WG, Cloud GA, Robinson P, Grieco MH, Sharkey PK, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS‐associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. New England Journal of Medicine 1992;326(2):83‐9. - PubMed
Saag 1999 {published data only}
-
- Saag MS, Cloud GA, Graybill JR, Sobel JD, Tuazon CU, Johnson PC, et al. A comparison of itraconazole versus fluconazole as maintenance therapy for AIDS‐associated cryptococcal meningitis. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clinical Infectious Diseases 1999;28(2):291‐6. - PubMed
Sharkey 1996 {published data only}
-
- Sharkey PK, Graybill JR, Johnson ES, Hausrath SG, Pollard RB, Kolokathis A. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clinical Infectious Diseases 1996;22(2):315‐21. - PubMed
Tansuphaswadikul 2006 {published data only}
-
- Tansuphaswadikul S, Maek‐a‐Nantawat W, Phonrat B, Boonpokbn L, Mctm AG, Pitisuttithum P. Comparison of one week with two week regimens of amphotericin B both followed by fluconazole in the treatment of cryptococcal meningitis among AIDS patients. Journal of the Medical Association of Thailand 2006;89(10):1677‐85. - PubMed
Techapornroong 2007 {published data only}
-
- Techapornroong M, Suankratay C. Alternate‐day versus once‐daily administration of amphotericin B in the treatment of cryptococcal meningitis: a randomized controlled trial. Scandinavian Journal of Infectious Diseases 2007;39(10):896‐901. - PubMed
Vaidhya 2015 {published data only}
Vibhagool 2003 {published data only}
-
- Vibhagool A, Sungkanuparph S, Mootsikapun P, Chetchotisakd P, Tansuphaswaswadikul S, Bowonwatanuwong C. Discontinuation of secondary prophylaxis for cryptococcal meningitis in human immunodeficiency virus‐infected patients treated with highly active antiretroviral therapy: a prospective, multicenter, randomized study. Clinical Infectious Diseases 2003;36(10):1329‐31. - PubMed
Villanueva‐Lozano 2018 {published data only}
-
- Villanueva‐Lozano H, Trevino‐Rangel RJ, Gonzalez GM, Hernandez‐Rodriguez PA, Camacho‐Ortiz A, Castillo‐Reyna L, et al. Clinical evaluation of the antifungal effect of sertraline in the treatment of cryptococcal meningitis in HIV patients: a single Mexican center experience. Infection 2018;46(1):25‐30. - PubMed
References to ongoing studies
ISRCTN72509687 {unpublished data only}
-
- ISRCTN72509687. High dose AMBISOME on a fluconazole backbone for cryptococcal meningitis induction therapy in sub‐Saharan Africa. www.isrctn.com/ISRCTN72509687 (first received 23 June 2017).
NCT00885703 {unpublished data only}
-
- NCT00885703. High‐dose fluconazole for the treatment of cryptococcal meningitis in HIV‐infected individuals (HiFLAC) [A phase I/II dose‐finding study of high‐dose fluconazole treatment in AIDS‐associated cryptococcal meningitis]. clinicaltrials.gov/ct2/show/NCT00885703 (first received 22 April 2009).
NCT01802385 {unpublished data only}
-
- NCT01802385. Adjunctive sertraline for the treatment of HIV‐associated cryptococcal meningitis (ASTRO‐CM). clinicaltrials.gov/ct2/show/NCT01802385 (first received 1 March 2013).
Additional references
Anderegg 2017
-
- Anderegg N, Kirk O on behalf of IeDEA‐Global Adults and COHERE. Immunodeficiency at the start of combination antiretroviral therapy in low‐, middle‐ and high‐income countries. 9th International AIDS Society Conference on HIV Science; 2017 July 23‐26; Paris, France. 2017.
Bahr 2014
-
- Bahr NC, Rolfes MA, Musubire A, Nabeta H, Williams DA, Rhein J, et al. Standardized electrolyte supplementation and fluid management improves survival during amphotericin therapy for cryptococcal meningitis in resource‐limited settings. Open Forum Infectious Diseases 2014;1(2):ofu070. - PMC - PubMed
Bicanic 2006
-
- Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV‐associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clinical Infectious Diseases 2006;43(8):1069‐73. - PubMed
Bicanic 2007
-
- Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral‐naive or antiretroviral‐experienced patients treated with amphotericin B or fluconazole. Clinical Infectious Diseases 2007;45(1):76‐80. - PubMed
Bicanic 2009
-
- Bicanic T, Brouwer AE, Meintjes G, Rebe K, Limmathurotsakul D, Chierakul W, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS 2009;23(6):701‐6. - PubMed
Bicanic 2015
Boulware 2014a
Campbell 2015
Chaimani 2013
Chen 2000
-
- Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, et al. Epidemiology and host‐ and variety‐dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clinical Infectious Diseases 2000;31(2):499‐508. - PubMed
CINeMA 2017
-
- CINeMA. Confidence in Network Meta‐Analysis [Software]. Institute of Social and Preventive Medicine, University of Bern 2017. Available from cinema.ispm.ch.
DAIDS 2014
-
- U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, Version 2.0. rsc.tech‐res.com/docs/default‐source/safety/daids_ae_grading_table_v2_nov2014.pdf 2014 (accessed prior to 10 June 2018).
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. - PubMed
Dromer 2004
-
- Dromer F, Mathoulin‐Pélissier S, Fontanet A, Ronin O, Dupont B, Lortholary O, et al. Epidemiology of HIV‐associated cryptococcosis in France (1985‐2001): comparison of the pre‐ and post‐HAART eras. AIDS 2004;18(3):555‐62. - PubMed
EndNote 2013 [Computer program]
-
- Clavirate Analytics. EndNote. Version Endnote X7 for Mac. Clavirate Analytics, July 2013.
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
Jarvis 2014
Livermore 2014
Longley 2008
-
- Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high‐dose fluconazole for HIV‐associated cryptococcal meningitis in southwestern Uganda. Clinical Infectious Diseases 2008;47(12):1556‐61. - PubMed
Loyse 2010
Loyse 2013a
Loyse 2013b
-
- Loyse A, Thangaraj H, Easterbrook P, Ford N, Roy M, Chiller T, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource‐poor countries. Lancet Infectious Diseases 2013;13(7):629‐37. - PubMed
Lu 2006
-
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101:447‐59.
Meda 2014
-
- Meda J, Kalluvya S, Downs JA, Chofle AA, Seni J, Kidenya B, et al. Cryptococcal meningitis management in Tanzania with strict schedule of serial lumber punctures using intravenous tubing sets: an operational research study. Journal of Acquired Immune Deficiency Syndromes 2014;66(2):e31‐6. - PubMed
Milefchik 2008
-
- Milefchik E, Leal MA, Haubrich R, Bozzette SA, Tilles JG, Leedom JM, et al. Fluconazole alone or combined with flucytosine for the treatment of AIDS‐associated cryptococcal meningitis. Medical Mycology 2008;46(4):393‐5. - PubMed
Moher 2009
Moosa 1997
-
- Moosa MY, Coovadia YM. Cryptococcal meningitis in Durban, South Africa: a comparison of clinical features, laboratory findings, and outcome for human immunodeficiency virus (HIV)‐positive and HIV‐negative patients. Clinical Infectious Diseases 1997;24(2):131‐4. - PubMed
Muzoora 2012
-
- Muzoora CK, Kabanda T, Ortu G, Ssentamu J, Hearn P, Mwesigye J, et al. Short course amphotericin B with high dose fluconazole for HIV‐associated cryptococcal meningitis. Journal of Infection 2012;64(1):76‐81. - PubMed
Perfect 2010
Pyrgos 2013
Rajasingham 2017
RevMan 2014 [Computer program]
-
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rolfes 2014
Rothe 2013
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. - PubMed
Salanti 2014
Schaars 2006
Scriven 2016
-
- Scriven JE, Lalloo DG, Meintjes G. Changing epidemiology of HIV‐associated cryptococcosis in sub‐Saharan Africa. Lancet Infectious Diseases 2016;16(8):891‐2. - PubMed
Sloan 2008
Stata 2013 [Computer program]
-
- StataCorp. Stata. Version 13.0. StataCorp, June 2013.
Vermes 2000
-
- Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. Journal of Antimicrobial Chemotherapy 2000;46(2):171‐9. - PubMed
White 2011
-
- White IR. Multivariate random‐effects meta‐regression: updates to mvmeta. Stata Journal 2011;11(2):255‐70.
White 2012
White 2015
-
- White IR. Network meta‐analysis. Stata Journal 2015;15(4):951‐85.
WHO 2011
-
- World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV‐infected adults, adolescents and children. www.who.int/hiv/pub/cryptococcal_disease2011/en/ (accessed 19 June 2018). - PubMed
WHO 2018
-
- World Health Organization. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV‐infected adults, adolescents and children. www.who.int/hiv/pub/guidelines/cryptococcal‐disease/en/ (accessed 19 June 2018). - PubMed
References to other published versions of this review
Sloan 2006
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

