CDK1 inhibition sensitizes normal cells to DNA damage in a cell cycle dependent manner
- PMID: 30045664
- PMCID: PMC6132956
- DOI: 10.1080/15384101.2018.1491236
CDK1 inhibition sensitizes normal cells to DNA damage in a cell cycle dependent manner
Abstract
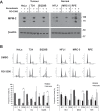
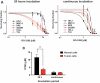
Cyclin-dependent kinase 1 (CDK1) orchestrates the transition from the G2 phase into mitosis and as cancer cells often display enhanced CDK1 activity, it has been proposed as a tumor specific anti-cancer target. Here we show that the effects of CDK1 inhibition are not restricted to tumor cells but can also reduce viability in non-cancer cells and sensitize them to radiation in a cell cycle dependent manner. Radiosensitization by the specific CDK1 inhibitor, RO-3306, was determined by colony formation assays in three tumor lines (HeLa, T24, SQ20B) and three non-cancer lines (HFL1, MRC-5, RPE). Initial results showed that CDK1 inhibition radiosensitized tumor cells, but did not sensitize normal fibroblasts and epithelial cells in colony formation assays despite effective inhibition of CDK1 signaling. Further investigation showed that normal cells were less sensitive to CDK1 inhibition because they remained predominantly in G1 for a prolonged period when plated in colony formation assays. In contrast, inhibiting CDK1 a day after plating, when the cells were going through G2/M phase, reduced their clonogenic survival both with and without radiation. Our finding that inhibition of CDK1 can damage normal cells in a cell cycle dependent manner indicates that targeting CDK1 in cancer patients may lead to toxicity in normal proliferating cells. Furthermore, our finding that cell cycle progression becomes easily stalled in non-cancer cells under normal culture conditions has general implications for testing anti-cancer agents in these cells.
Keywords: CDK1 (cyclin-dependent kinase) inhibitor; RO-3306; cell cycle arrest; epithelial cells; fibroblasts; radiation; radiosensitivity; radiotherapy.
Figures



References
-
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001. January;2(1):21–32. PubMed PMID: 11413462. - PubMed
-
- Perez de Castro I, de Carcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007. May;28(5):899–912. PubMed PMID: 17259655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
