Antitumor activity of CAR-T cells targeting the intracellular oncoprotein WT1 can be enhanced by vaccination
- PMID: 30045840
- PMCID: PMC6148344
- DOI: 10.1182/blood-2017-08-802926
Antitumor activity of CAR-T cells targeting the intracellular oncoprotein WT1 can be enhanced by vaccination
Abstract
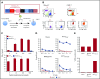
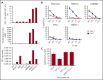
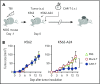
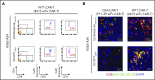
The recent success of chimeric antigen receptor (CAR)-T cell therapy for treatment of hematologic malignancies supports further development of treatments for both liquid and solid tumors. However, expansion of CAR-T cell therapy is limited by the availability of surface antigens specific for the tumor while sparing normal cells. There is a rich diversity of tumor antigens from intracellularly expressed proteins that current and conventional CAR-T cells are unable to target. Furthermore, adoptively transferred T cells often suffer from exhaustion and insufficient expansion, in part, because of the immunosuppressive mechanisms operating in tumor-bearing hosts. Therefore, it is necessary to develop means to further activate and expand those CAR-T cells in vivo. The Wilms tumor 1 (WT1) is an intracellular oncogenic transcription factor that is an attractive target for cancer immunotherapy because of its overexpression in a wide range of leukemias and solid tumors, and a low level of expression in normal adult tissues. In the present study, we developed CAR-T cells consisting of a single chain variable fragment (scFv) specific to the WT1235-243/HLA-A*2402 complex. The therapeutic efficacy of our CAR-T cells was demonstrated in a xenograft model, which was further enhanced by vaccination with dendritic cells (DCs) loaded with the corresponding antigen. This enhanced efficacy was mediated, at least partly, by the expansion and activation of CAR-T cells. CAR-T cells shown in the present study not only demonstrate the potential to expand the range of targets available to CAR-T cells, but also provide a proof of concept that efficacy of CAR-T cells targeting peptide/major histocompatibility complex can be boosted by vaccination.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: Y. Amaishi, S. Okamoto, and J.M. are employees of Takara Bio Inc. which collaborated in the development of virus vector construction. The remaining authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

