Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma
- PMID: 30045880
- PMCID: PMC6580753
- DOI: 10.1136/gutjnl-2017-315084
Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma
Abstract
Objective: To characterise gut microbiome in patients with hepatocellular carcinoma (HCC) and evaluate the potential of microbiome as non-invasive biomarkers for HCC.
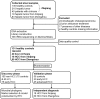
Design: We collected 486 faecal samples from East China, Central China and Northwest China prospectively and finally 419 samples completed Miseq sequencing. We characterised gut microbiome, identified microbial markers and constructed HCC classifier in 75 early HCC, 40 cirrhosis and 75 healthy controls. We validated the results in 56 controls, 30 early HCC and 45 advanced HCC. We further verified diagnosis potential in 18 HCC from Xinjiang and 80 HCC from Zhengzhou.
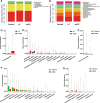
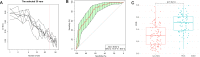
Results: Faecal microbial diversity was increased from cirrhosis to early HCC with cirrhosis. Phylum Actinobacteria was increased in early HCC versus cirrhosis. Correspondingly, 13 genera including Gemmiger and Parabacteroides were enriched in early HCC versus cirrhosis. Butyrate-producing genera were decreased, while genera producing-lipopolysaccharide were increased in early HCC versus controls. The optimal 30 microbial markers were identified through a fivefold cross-validation on a random forest model and achieved an area under the curve of 80.64% between 75 early HCC and 105 non-HCC samples. Notably, gut microbial markers validated strong diagnosis potential for early HCC and even advanced HCC. Importantly, microbial markers successfully achieved a cross-region validation of HCC from Northwest China and Central China.
Conclusions: This study is the first to characterise gut microbiome in patients with HCC and to report the successful diagnosis model establishment and cross-region validation of microbial markers for HCC. Gut microbiota-targeted biomarkers represent potential non-invasive tools for early diagnosis of HCC.
Keywords: early diagnosis; gut microbiota; hepatocellular carcinoma; liver cirrhosis.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
