LRRK2 activation in idiopathic Parkinson's disease
- PMID: 30045977
- PMCID: PMC6344941
- DOI: 10.1126/scitranslmed.aar5429
LRRK2 activation in idiopathic Parkinson's disease
Abstract
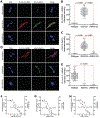
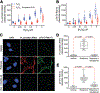
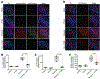
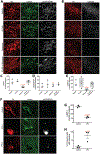
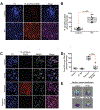
Missense mutations in leucine-rich repeat kinase 2 (LRRK2) cause familial Parkinson's disease (PD). However, a potential role of wild-type LRRK2 in idiopathic PD (iPD) remains unclear. Here, we developed proximity ligation assays to assess Ser1292 phosphorylation of LRRK2 and, separately, the dissociation of 14-3-3 proteins from LRRK2. Using these proximity ligation assays, we show that wild-type LRRK2 kinase activity was selectively enhanced in substantia nigra dopamine neurons in postmortem brain tissue from patients with iPD and in two different rat models of the disease. We show that this occurred through an oxidative mechanism, resulting in phosphorylation of the LRRK2 substrate Rab10 and other downstream consequences including abnormalities in mitochondrial protein import and lysosomal function. Our study suggests that, independent of mutations, wild-type LRRK2 plays a role in iPD. LRRK2 kinase inhibitors may therefore be useful for treating patients with iPD who do not carry LRRK2 mutations.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing interests: Greenamyre briefly held an advisory position at Pfizer. Otherwise, the authors declare that they have no competing financial interests.
Figures



References
-
- Kalia LV, Lang AE, Parkinson disease in 2015: Evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol 12, 65–66 (2016). - PubMed
-
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB, Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44, 595–600 (2004). - PubMed
-
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T, Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 (2004). - PubMed
-
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T, Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41, 1308–1312 (2009). - PMC - PubMed
-
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR, Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis 23, 329–341 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

