Exogenous LRRK2G2019S induces parkinsonian-like pathology in a nonhuman primate
- PMID: 30046008
- PMCID: PMC6124435
- DOI: 10.1172/jci.insight.98202
Exogenous LRRK2G2019S induces parkinsonian-like pathology in a nonhuman primate
Abstract
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease among the elderly. To understand its pathogenesis and to test therapies, animal models that faithfully reproduce key pathological PD hallmarks are needed. As a prelude to developing a model of PD, we tested the tropism, efficacy, biodistribution, and transcriptional effect of canine adenovirus type 2 (CAV-2) vectors in the brain of Microcebus murinus, a nonhuman primate that naturally develops neurodegenerative lesions. We show that introducing helper-dependent (HD) CAV-2 vectors results in long-term, neuron-specific expression at the injection site and in afferent nuclei. Although HD CAV-2 vector injection induced a modest transcriptional response, no significant adaptive immune response was generated. We then generated and tested HD CAV-2 vectors expressing leucine-rich repeat kinase 2 (LRRK2) and LRRK2 carrying a G2019S mutation (LRRK2G2019S), which is linked to sporadic and familial autosomal dominant forms of PD. We show that HD-LRRK2G2019S expression induced parkinsonian-like motor symptoms and histological features in less than 4 months.
Keywords: Neurodegeneration; Neuroscience; Parkinson’s disease.
Conflict of interest statement
Figures

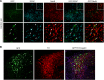
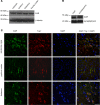
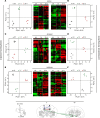
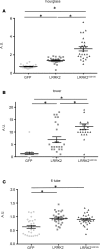
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

