Cachexia-associated adipose loss induced by tumor-secreted leukemia inhibitory factor is counterbalanced by decreased leptin
- PMID: 30046014
- PMCID: PMC6124433
- DOI: 10.1172/jci.insight.121221
Cachexia-associated adipose loss induced by tumor-secreted leukemia inhibitory factor is counterbalanced by decreased leptin
Abstract
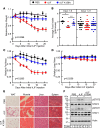
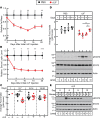
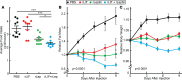
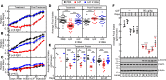
Cachexia syndrome consists of adipose and muscle loss, often despite normal food intake. We hypothesized that cachexia-associated adipose wasting is driven in part by tumor humoral factors that induce adipocyte lipolysis. We developed an assay to purify secreted factors from a cachexia-inducing colon cancer line that increases lipolysis in adipocytes and identified leukemia inhibitory factor (LIF) by mass spectrometry. Recombinant LIF induced lipolysis in vitro. Peripheral LIF administered to mice caused >50% loss of adipose tissue and >10% reduction in body weight despite only transient hypophagia due to decreasing leptin. LIF-injected mice lacking leptin (ob/ob) resulted in persistent hypophagia and loss of adipose tissue and body weight. LIF's peripheral role of initiating lipolysis in adipose loss was confirmed in pair-fed ob/ob mouse studies. Our studies demonstrate that (a) LIF is a tumor-secreted factor that promotes cachexia-like adipose loss when administered peripherally, (b) LIF directly induces adipocyte lipolysis, (c) LIF has the ability to sustain adipose and body weight loss through an equal combination of peripheral and central contributions, and (d) LIF's central effect is counterbalanced by decreased leptin signaling, providing insight into cachexia's wasting, despite normophagia.
Keywords: Adipose tissue; Cytokines; Leptin; Metabolism; Oncology.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

