Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss
- PMID: 30046091
- PMCID: PMC6060116
- DOI: 10.1038/s41467-018-05244-y
Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss
Abstract
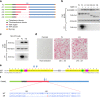
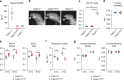
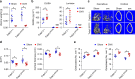
Receptor activator of NFkB ligand (RANKL) is a TNF-family cytokine required for osteoclast formation, as well as immune cell and mammary gland development. It is produced as a membrane-bound protein that can be shed to form a soluble protein. We created mice harboring a sheddase-resistant form of RANKL, in which soluble RANKL is undetectable in the circulation. Lack of soluble RANKL does not affect bone mass or structure in growing mice but reduces osteoclast number and increases cancellous bone mass in adult mice. Nonetheless, the bone loss caused by estrogen deficiency is unaffected by the lack of soluble RANKL. Lymphocyte number, lymph node development, and mammary gland development are also unaffected by the absence of soluble RANKL. These results demonstrate that the membrane-bound form of RANKL is sufficient for most functions of this protein but that the soluble form does contribute to physiological bone remodeling in adult mice.
Conflict of interest statement
C.A.O. owns stock in Radius Health, Inc. The remaining authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

