CAR T cells for infection, autoimmunity and allotransplantation
- PMID: 30046149
- PMCID: PMC6505691
- DOI: 10.1038/s41577-018-0042-2
CAR T cells for infection, autoimmunity and allotransplantation
Abstract
Chimeric antigen receptors (CARs) have shown remarkable ability to re-direct T cells to target CD19-expressing tumours, resulting in remission rates of up to 90% in individuals with paediatric acute lymphoblastic lymphoma. Lessons learned from these clinical trials of adoptive T cell therapy for cancer, as well as investments made in manufacturing T cells at commercial scale, have inspired researchers to develop CARs for additional applications. Here, we explore the challenges and opportunities of using this technology to target infectious diseases such as with HIV and undesired immune responses such as autoimmunity and transplant rejection. Despite substantial obstacles, the potential of CAR T cells to enable cures for a wide array of disease settings could be transformational for the medical field.
Conflict of interest statement
Competing interests
J.L.R. holds equity in Tmunity Therapeutics. The other authors declare no competing interests.
Figures
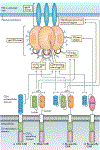


References
-
- McMichael A T cell responses and viral escape. Cell 93, 673–676 (1998). - PubMed
-
- Roberts MR et al. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood 84, 2878–2889 (1994). - PubMed
-
- Mitsuyasu RT et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood 96, 785–793 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

