Design and rationale for the DIVERSITY study: An open-label, randomized study of dabigatran etexilate for pediatric venous thromboembolism
- PMID: 30046738
- PMCID: PMC6055566
- DOI: 10.1002/rth2.12086
Design and rationale for the DIVERSITY study: An open-label, randomized study of dabigatran etexilate for pediatric venous thromboembolism
Abstract
Background: The current standard of care (SOC) for pediatric venous thromboembolism (VTE) comprises unfractionated heparin (UFH), or low-molecular-weight heparin (LMWH) followed by LMWH or vitamin K antagonists, all of which have limitations. Dabigatran etexilate (DE) has demonstrated efficacy and safety for adult VTE and has the potential to overcome some of the limitations of the current SOC. Pediatric trials are needed to establish dosing in children and to confirm that results obtained in adults are applicable in the pediatric setting.
Objectives: To describe the design and rationale of a planned phase IIb/III trial that will evaluate a proposed dosing algorithm for DE and assess the safety and efficacy of DE versus SOC for pediatric VTE treatment.
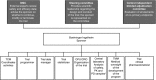
Patients/methods: An open-label, randomized, parallel-group noninferiority study will be conducted in approximately 180 patients aged 0 to <18 years with VTE, who have received initial UFH or LMWH treatment and who are expected to require ≥3 months of anticoagulation therapy. Patients will receive DE or SOC for 3 months. DE will be administered twice daily as capsules, pellets, or an oral liquid formulation according to patient age. Initial doses will be calculated using a proposed dosing algorithm.
Results: There will be two coprimary endpoints: a composite efficacy endpoint comprising the proportion of patients with complete thrombus resolution, freedom from recurrent VTE and VTE-related mortality, and a safety endpoint: freedom from major bleeding events.
Conclusion: Findings will provide valuable information regarding the efficacy and safety of DE for the treatment of pediatric VTE. ClinicalTrials.gov registration number: NCT01895777.
Keywords: anticoagulants; dabigatran etexilate; direct thrombin inhibitors; pediatrics; venous thromboembolism.
Figures
References
-
- Goldenberg NA. Long‐term outcomes of venous thrombosis in children. Curr Opin Hematol. 2005;12:370–6. - PubMed
-
- Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83:1251–7. - PubMed
-
- van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two‐year registry in the Netherlands. J Pediatr. 2001;139:676–81. - PubMed
-
- Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–8. - PubMed
-
- Chan AK, Monagle P. Updates in thrombosis in pediatrics: where are we after 20 years? Hematology Am Soc Hematol Educ Program. 2012;2012:439–43. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

