[18F]Fluciclovine PET discrimination between high- and low-grade gliomas
- PMID: 30046944
- PMCID: PMC6060188
- DOI: 10.1186/s13550-018-0415-3
[18F]Fluciclovine PET discrimination between high- and low-grade gliomas
Abstract
Background: The ability to accurately and non-invasively distinguish high-grade glioma from low-grade glioma remains a challenge despite advances in molecular and magnetic resonance imaging. We investigated the ability of fluciclovine (18F) PET as a means to identify and distinguish these lesions in patients with known gliomas and to correlate uptake with Ki-67.
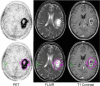
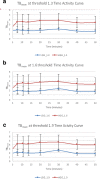
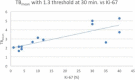
Results: Sixteen patients with a total of 18 newly diagnosed low-grade gliomas (n = 6) and high grade gliomas (n = 12) underwent fluciclovine PET imaging after histopathologic assessment. Fluciclovine PET analysis comprised tumor SUVmax and SUVmean, as well as metabolic tumor thresholds (1.3*, 1.6*, 1.9*) to normal brain background (TBmax, and TBmean). Comparison was additionally made to the proliferative status of the tumor as indicated by Ki-67 values. Fluciclovine uptake greater than normal brain parenchyma was found in all lesions studied. Time activity curves demonstrated statistically apparent flattening of the curves for both high-grade gliomas and low-grade gliomas starting 30 min after injection, suggesting an influx/efflux equilibrium. The best semiquantitative metric in discriminating HGG from LGG was obtained utilizing a metabolic 1 tumor threshold of 1.3* contralateral normal brain parenchyma uptake to create a tumor: background (TBmean1.3) cutoff of 2.15 with an overall sensitivity of 97.5% and specificity of 95.5%. Additionally, using a SUVmax > 4.3 cutoff gave a sensitivity of 90.9% and specificity of 97.5%. Tumor SUVmean and tumor SUVmax as a ratio to mean normal contralateral brain were both found to be less relevant predictors of tumor grade. Both SUVmax (R = 0.71, p = 0.0227) and TBmean (TBmean1.3: R = 0.81, p = 0.00081) had a high correlation with the tumor proliferative index Ki-67.
Conclusions: Fluciclovine PET produces high-contrast images between both low-grade and high grade gliomas and normal brain by visual and semiquantitative analysis. Fluciclovine PET appears to discriminate between low-grade glioma and high-grade glioma, but must be validated with a larger sample size.
Keywords: 18F-fluciclovine; Amino acid; Glioma; PET.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The recruitment protocol was approved by the Institutional Review Board (IRB) and complied with the Health Insurance Portability and Accountability Act (HIPPA). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors have participated in sponsored research involving 18F-fluciclovine, among other radiotracers. Emory University and Dr. Mark Goodman are eligible to receive royalties for 18F-fluciclovine. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Approvals SODDa. U.S. Food & Drug Administration website. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm. Accessed 30 May 2017.
-
- Sasajima T, Ono T, Shimada N, Doi Y, Oka S, Kanagawa M, et al. Trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid (anti-18F-FACBC) is a feasible alternative to 11C-methyl-L-methionine and magnetic resonance imaging for monitoring treatment response in gliomas. Nucl Med Biol. 2013;40:808–15. 10.1016/j.nucmedbio.2013.04.007. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

