Protecting Encapsulin Nanoparticles with Cysteine-Knot Miniproteins
- PMID: 30047270
- PMCID: PMC6143317
- DOI: 10.1021/acs.molpharmaceut.8b00630
Protecting Encapsulin Nanoparticles with Cysteine-Knot Miniproteins
Abstract
A big hurdle for the use of protein-based drugs is that they are easily degraded by proteases in the human body. In an attempt to solve this problem, we show the possibility to functionalize TM encapsulin nanoparticles with an mEETI-II knottin miniprotein from the cysteine-stabilized knot class. The resulting particles did not show aggregation and retained part of their protease inhibitive function. This imposes a protection toward protease, in this case, trypsin, degradation of the protein cage. The used chemistry is easy to apply and thus suitable to protect other protein systems from degradation. In addition, this proof of principle opens up the use of other knottins or cysteine-stabilized knots, which can be attached to protein cages to create a heterofunctionalized protein nanocage. This allows specific targeting and tumor suppression among other types of functionalization. Overall, this is a promising strategy to protect a protein of interest which brings oral administration of protein-based drugs one step closer.
Keywords: antibody substitute; bacterial nanocompartment; nanocage; protease inhibitor.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
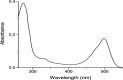
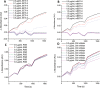

References
-
- Kirkegaard T.; Gray J.; Priestman D. A.; Wallom K. L.; Atkins J.; Olsen O. D.; Klein A.; Drndarski S.; Petersen N. H.; Ingemann L.; Smith D. A.; Morris L.; Bornæs C.; Jørgensen S. H.; Williams I.; Hinsby A.; Arenz C.; Begley D.; Jäättelä M.; Platt F. M. Heat Shock Protein-based Therapy as a Potential Candidate for Treating the Sphingolipidoses. Sci. Transl. Med. 2016, 8, 355ra118.10.1126/scitranslmed.aad9823. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

