Repeated Intrathecal Mesenchymal Stem Cells for Amyotrophic Lateral Sclerosis
- PMID: 30048006
- PMCID: PMC6175096
- DOI: 10.1002/ana.25302
Repeated Intrathecal Mesenchymal Stem Cells for Amyotrophic Lateral Sclerosis
Abstract
Objective: To assess the safety and efficacy of 2 repeated intrathecal injections of autologous bone marrow-derived mesenchymal stem cells (BM-MSCs) in amyotrophic lateral sclerosis (ALS).
Methods: In a phase 2 randomized controlled trial (NCT01363401), 64 participants with ALS were randomly assigned treatments (1:1) of riluzole alone (control group, n = 31) or combined with 2 BM-MSC injections (MSC group, n = 33). Safety was assessed based on the occurrence of adverse events. The primary efficacy outcome was changes in Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) score from baseline to 4 and 6 months postinjection. Post hoc analysis includes investigation of cerebrospinal fluid biomarkers and long-term survival analysis.
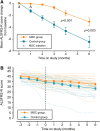
Results: Safety rating showed no groupwise difference with absence of serious treatment-related adverse events. Mean changes in ALSFRS-R scores from baseline to 4 and 6 months postinjection were reduced in the MSC group compared with the control group (4 months: 2.98, 95% confidence interval [CI] = 1.48-4.47, p < 0.001; 6 months: 3.38, 95% CI = 1.23-5.54, p = 0.003). The MSC group showed decreased proinflammatory and increased anti-inflammatory cytokines. In good responders, transforming growth factor β1 significantly showed inverse correlation with monocyte chemoattractant protein-1. There was no significant difference in long-term survival between groups.
Interpretation: Repeated intrathecal injections of BM-MSCs demonstrated a possible clinical benefit lasting at least 6 months, with safety, in ALS patients. A plausible action mechanism is that BM-MSCs mediate switching from pro- to anti-inflammatory conditions. A future randomized, double-blind, large-scale phase 3 clinical trial with additional BM-MSC treatments is required to evaluate long-term efficacy and safety. Ann Neurol 2018;84:361-373.
© 2018 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures


References
-
- Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet 2011;377:942–955. - PubMed
-
- Sreedharan J, Brown RH. Amyotrophic lateral sclerosis: problems and prospects. Ann Neurol 2013;74:309–316. - PubMed
-
- Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol 2011;10:649–656. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

