Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: A randomized clinical trial
- PMID: 30048473
- PMCID: PMC6061993
- DOI: 10.1371/journal.pone.0200727
Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: A randomized clinical trial
Abstract
Objective: Evaluate and compare the isolated and combined effects of Inspiratory Muscle Training (IMT) and Aerobic Training (AT) on respiratory and functional parameters, inflamatory biomarkers, redox status and health-related quality of life (HRQoL) in hemodialysis patients.
Methods: A randomised controlled trial with factorial allocation and intention-to-treat analysis was performed in hemodialysis patients. Volunteers were randomly assigned to performe 8-weeks of IMT at 50% of maximal inspiratory pressure (MIP), low intensity AT or combined training (CT). Before the interventions, all the volunteers went 8-weeks through a control period (without training). Measures are taken at baseline, 8-week (after control period) and 16-week (after the interventions). Primary outcomes were functional capacity (incremental shuttle walk test), MIP and lower limbs strength (Sit-to-Stand test of 30 seconds). Plasma levels of interleukin-6 (IL-6), soluble tumor necrosis factor receptor 1 (sTNFR1) and 2 (sTNFR2), adiponectin, resistin and leptin, redox status parameters and HRQoL (KDQOL-SF questionnaire) were the scondary outcomes. Data analyses were performed by two-way repeated measurements ANOVA.
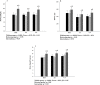
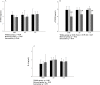
Results: 37 hemodialysis patients aged 48.2 years old (IC95% 43.2-54.7) were randomized. Increase of MIP, functional capacity, lower limbs strength and resistin levels, and reduction of sTNFR2 levels in 16-week, compared to baseline and 8-week, were observed in all the groups (p<0.001). IMT improved functional capacity, MIP and lower limbs strength in 96.7m (IC95% 5.6-189.9), 34.5cmH2O (IC95% 22.4-46.7) and 2.2repetitions (IC95% 1.1-3.2) respectively. Increase in resistin leves and reduction in sTNFR2 leves after IMT was 0.8ng/dL (IC95% 0.5-1.1) and 0.8ng/dL (IC95% 0.3-1.3), respectively, without between-group differences. Compared to baseline and 8-week, adiponectin levels (p<0.001) and fatigue domain of the HRQoL (p<0.05) increased in 16-week only in CT.
Conclusion: IMT, AT and CT improved functional parameters and modulated inflammatory biomarkers, in addition, IMT provoked a similar response to low intensity AT in hemodialysis patients.
Trial registration: Registro Brasileiro de Ensaios clínicos RBR-4hv9rs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

