C-type natriuretic peptide analog treatment of craniosynostosis in a Crouzon syndrome mouse model
- PMID: 30048539
- PMCID: PMC6062116
- DOI: 10.1371/journal.pone.0201492
C-type natriuretic peptide analog treatment of craniosynostosis in a Crouzon syndrome mouse model
Abstract
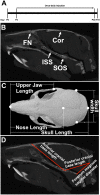
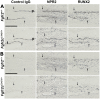
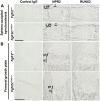
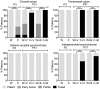
Activating mutations of fibroblast growth factor receptors (FGFRs) are a major cause of skeletal dysplasias, and thus they are potential targets for pharmaceutical intervention. BMN 111, a C-type natriuretic peptide analog, inhibits FGFR signaling at the level of the RAF1 kinase through natriuretic peptide receptor 2 (NPR2) and has been shown to lengthen the long bones and improve skull morphology in the Fgfr3Y367C/+ thanatophoric dysplasia mouse model. Here we report the effects of BMN 111 in treating craniosynostosis and aberrant skull morphology in the Fgfr2cC342Y/+ Crouzon syndrome mouse model. We first demonstrated that NPR2 is expressed in the murine coronal suture and spheno-occipital synchondrosis in the newborn period. We then gave Fgfr2cC342Y/+ and Fgfr2c+/+ (WT) mice once-daily injections of either vehicle or reported therapeutic levels of BMN 111 between post-natal days 3 and 31. Changes in skeletal morphology, including suture patency, skull dimensions, and long bone length, were assessed by micro-computed tomography. Although BMN 111 treatment significantly increased long bone growth in both WT and mutant mice, skull dimensions and suture patency generally were not significantly affected. A small but significant increase in the relative length of the anterior cranial base was observed. Our results indicate that the differential effects of BMN 111 in treating various skeletal dysplasias may depend on the process of bone formation targeted (endochondral or intramembranous), the specific FGFR mutated, and/or the specific signaling pathway changes due to a given mutation.
Conflict of interest statement
LZ, RM, LS, and TO are employees of BioMarin Pharmaceutical, Inc., that sponsored this study. GH and EWJ received funding from BioMarin, but do not have any commercial interest in BioMarin Pharmaceutical, Inc. This does not alter the authors’ adherence to PLOS ONE policies on sharing data and materials. The internal publication review committee of BioMarin Pharmaceutical, Inc., approved the manuscript for publication.
Figures


References
-
- Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, et al. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989; 58(6):1155–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

