Representation, Pattern Information, and Brain Signatures: From Neurons to Neuroimaging
- PMID: 30048614
- PMCID: PMC6296466
- DOI: 10.1016/j.neuron.2018.06.009
Representation, Pattern Information, and Brain Signatures: From Neurons to Neuroimaging
Abstract
Human neuroimaging research has transitioned from mapping local effects to developing predictive models of mental events that integrate information distributed across multiple brain systems. Here we review work demonstrating how multivariate predictive models have been utilized to provide quantitative, falsifiable predictions; establish mappings between brain and mind with larger effects than traditional approaches; and help explain how the brain represents mental constructs and processes. Although there is increasing progress toward the first two of these goals, models are only beginning to address the latter objective. By explicitly identifying gaps in knowledge, research programs can move deliberately and programmatically toward the goal of identifying brain representations underlying mental states and processes.
Keywords: affect; brain signature; decoding; fMRI; machine learning; multivariate; pain; pattern recognition; population coding; representation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
T.D.W. has the following patents related to this work:
1. US 2016/0054409 fMRI-based Neurologic Signature of Physical Pain (PCT/US14/33538)
2. US 2018/0055407 Neurophysiological signatures for fibromyalgia (CU4199B-PPA1).
Figures
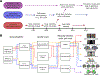


Similar articles
-
Transfer learning of deep neural network representations for fMRI decoding.J Neurosci Methods. 2019 Dec 1;328:108319. doi: 10.1016/j.jneumeth.2019.108319. Epub 2019 Oct 1. J Neurosci Methods. 2019. PMID: 31585315
-
Pushing the Limits of Human Neuroimaging.JAMA. 2015 Sep 8;314(10):993-4. doi: 10.1001/jama.2015.10229. JAMA. 2015. PMID: 26348745 No abstract available.
-
Decoding the Nature of Emotion in the Brain.Trends Cogn Sci. 2016 Jun;20(6):444-455. doi: 10.1016/j.tics.2016.03.011. Epub 2016 Apr 25. Trends Cogn Sci. 2016. PMID: 27133227 Free PMC article. Review.
-
Graph-based inter-subject pattern analysis of FMRI data.PLoS One. 2014 Aug 15;9(8):e104586. doi: 10.1371/journal.pone.0104586. eCollection 2014. PLoS One. 2014. PMID: 25127129 Free PMC article.
-
Emotions as discrete patterns of systemic activity.Neurosci Lett. 2019 Feb 6;693:3-8. doi: 10.1016/j.neulet.2017.07.012. Epub 2017 Jul 10. Neurosci Lett. 2019. PMID: 28705730 Review.
Cited by
-
A generative model of electrophysiological brain responses to stimulation.Elife. 2024 Jan 17;12:RP87729. doi: 10.7554/eLife.87729. Elife. 2024. PMID: 38231034 Free PMC article.
-
Individual Differences in Cognitive Performance Are Better Predicted by Global Rather Than Localized BOLD Activity Patterns Across the Cortex.Cereb Cortex. 2021 Feb 5;31(3):1478-1488. doi: 10.1093/cercor/bhaa290. Cereb Cortex. 2021. PMID: 33145600 Free PMC article.
-
The Neural Separability of Emotion Reactivity and Regulation.Affect Sci. 2023 Dec 4;4(4):617-629. doi: 10.1007/s42761-023-00227-9. eCollection 2023 Dec. Affect Sci. 2023. PMID: 38156247 Free PMC article.
-
Enhancing task fMRI individual difference research with neural signatures.medRxiv [Preprint]. 2025 Jan 31:2025.01.30.25321355. doi: 10.1101/2025.01.30.25321355. medRxiv. 2025. PMID: 39974058 Free PMC article. Preprint.
-
A neural signature for the subjective experience of threat anticipation under uncertainty.Nat Commun. 2024 Feb 20;15(1):1544. doi: 10.1038/s41467-024-45433-6. Nat Commun. 2024. PMID: 38378947 Free PMC article.
References
-
- Amedi A, Stern WM, Camprodon JA, Bermpohl F, Merabet L, Rotman S, Hemond C, Meijer P, and Pascual-Leone A (2007). Shape conveyed by visual-to-auditory sensory substitution activates the lateral occipital complex. Nat Neurosci 10, 687–689. - PubMed
-
- Antal A, Brepohl N, Poreisz C, Boros K, Csifcsak G, and Paulus W (2008). Transcranial direct current stimulation over somatosensory cortex decreases experimentally induced acute pain perception. The Clinical journal of pain 24, 56–63. - PubMed
-
- Averbeck BB, Latham PE, and Pouget A (2006). Neural correlations, population coding and computation. Nat Rev Neurosci 7, 358–366. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

