In vivo biodistribution and toxicology studies of cadmium-free indium-based quantum dot nanoparticles in a rat model
- PMID: 30048815
- PMCID: PMC6198065
- DOI: 10.1016/j.nano.2018.07.009
In vivo biodistribution and toxicology studies of cadmium-free indium-based quantum dot nanoparticles in a rat model
Abstract
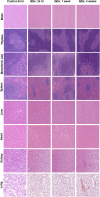
Quantum dot (QD) nanoparticles are highly promising contrast agents and probes for biomedical applications owing to their excellent photophysical properties. However, toxicity concerns about commonly used cadmium-based QDs hinder their translation to clinical applications. In this study we describe the in vivo biodistribution and toxicology of indium-based water soluble QDs in rats following intravenous administration. The biodistribution measured at up to 90 days showed that QDs mainly accumulated in the liver and spleen, with similar elimination kinetics to subcutaneous administration. Evidence for QD degradation in the liver was found by comparing photoluminescence measurements versus elemental analysis. No organ damage or histopathological lesions were observed for the QDs treated rats after 24 h, 1 and 4 weeks following intravenous administration at 12.5 mg/kg or 50 mg/kg. Analysis of serum biochemistry and complete blood counts found no toxicity. This work supports the strong potential of indium-based QDs for translation into the clinic.
Keywords: Biodistribution; Cadmium-free quantum dots; Nanoparticles; Toxicology.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Resch-Genger U., Grabolle M., Cavaliere-Jaricot S., Nitschke R., Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5:763–775. - PubMed
-
- Yaghini E., Seifalian A.M., MacRobert A.J. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine. 2009;4:353–363. - PubMed
-
- Chen N., He Y., Su Y., Li X., Huang Q., Wang H. The cytotoxicity of cadmium-based quantum dots. Biomaterials. 2012;33:1238–1244. - PubMed
-
- Li J., Chang Z., Chen X., Gu Z., Zhao F., Chai Z. Toxicity of inorganic nanomaterials in biomedical imaging. Biotechnol Adv. 2014;32:727–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

