STIM- and Orai-mediated calcium entry controls NF-κB activity and function in lymphocytes
- PMID: 30048879
- PMCID: PMC6415950
- DOI: 10.1016/j.ceca.2018.07.003
STIM- and Orai-mediated calcium entry controls NF-κB activity and function in lymphocytes
Abstract
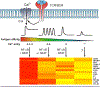
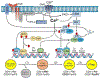
The central role of Ca2+ signaling in the development of functional immunity and tolerance is well established. These signals are initiated by antigen binding to cognate receptors on lymphocytes that trigger store operated Ca2+ entry (SOCE). The underlying mechanism of SOCE in lymphocytes involves TCR and BCR mediated activation of Stromal Interaction Molecule 1 and 2 (STIM1/2) molecules embedded in the ER membrane leading to their activation of Orai channels in the plasma membrane. STIM/Orai dependent Ca2+ signals guide key antigen induced lymphocyte development and function principally through direct regulation of Ca2+ dependent transcription factors. The role of Ca2+ signaling in NFAT activation and signaling is well known and has been studied extensively, but a wide appreciation and mechanistic understanding of how Ca2+ signals also shape the activation and specificity of NF-κB dependent gene expression has lagged. Here we discuss and interpret what is known about Ca2+ dependent mechanisms of NF-kB activation, including what is known and the gaps in our understanding of how these signals control lymphocyte development and function.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
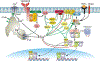
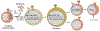
References
-
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006;441(7090):179–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

