Lipid imaging for visualizing cilastatin amelioration of cisplatin-induced nephrotoxicity
- PMID: 30049708
- PMCID: PMC6121926
- DOI: 10.1194/jlr.M080465
Lipid imaging for visualizing cilastatin amelioration of cisplatin-induced nephrotoxicity
Abstract
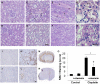
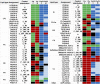
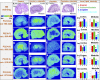
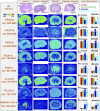
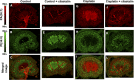
Nephrotoxicity is a major limitation to cisplatin antitumor therapies. Cilastatin, an inhibitor of renal dehydropeptidase-I, was recently proposed as a promising nephroprotector against cisplatin toxicity, preventing apoptotic cell death. In this work, cilastatin nephroprotection was further investigated in a rat model, with a focus on its effect on 76 renal lipids altered by cisplatin, including 13 new cisplatin-altered mitochondrial cardiolipin species. Lipid imaging was performed with MALDI mass spectrometry imaging (MALDI-MSI) in kidney sections from treated rats. Cilastatin was proved to significantly diminish the lipid distribution alterations caused by cisplatin, lipid levels being almost completely recovered to those of control samples. The extent of recovery of cisplatin-altered lipids by cilastatin turned out to be relevant for discriminating direct or secondary lipid alterations driven by cisplatin. Lipid peroxidation induced by cisplatin was also shown to be reduced when cilastatin was administered. Importantly, significant groups separation was achieved during multivariate analysis of cortex and outer-medullary lipids, indicating that damaged kidney can be discerned from the nephroprotected and healthy groups and classified according to lipid distribution. Therefore, we propose MALDI-MSI as a powerful potential tool offering multimolecule detection possibilities to visualize and evaluate nephrotoxicity and nephroprotection based on lipid analysis.
Keywords: cancer; kidney; mass spectrometry; molecular imaging; nephroprotection; renal disease.
Copyright © 2018 by the American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
A.L. and A.T. are coinventors of patents describing cilastatin as a nephroprotector against toxic injuries that are assigned to Fundación para la Investigación Biomédica Hospital Gregorio Marañón and licensed to Spherium Biomed S.L.
Figures


References
-
- Pabla N., and Dong Z.. 2008. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73: 994–1007. - PubMed
-
- Kelland L. 2007. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 7: 573–584. - PubMed
-
- Messori L., and Merlino A.. 2016. Cisplatin binding to proteins: a structural perspective. Coord. Chem. Rev. 315: 67–89.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

