The actin filament bundling protein α-actinin-4 actually suppresses actin stress fibers by permitting actin turnover
- PMID: 30049798
- PMCID: PMC6139541
- DOI: 10.1074/jbc.RA118.004345
The actin filament bundling protein α-actinin-4 actually suppresses actin stress fibers by permitting actin turnover
Abstract
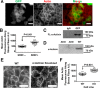
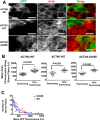
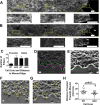
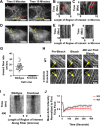
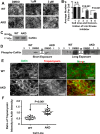
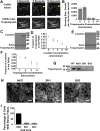
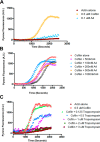
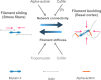
Cells organize actin filaments into contractile bundles known as stress fibers that resist mechanical stress, increase cell adhesion, remodel the extracellular matrix, and maintain tissue integrity. α-actinin is an actin filament bundling protein that is thought to be essential for stress fiber formation and stability. However, previous studies have also suggested that α-actinin might disrupt fibers, making the true function of this biomolecule unclear. Here we use fluorescence imaging to show that kidney epithelial cells depleted of α-actinin-4 via shRNA or CRISPR/Cas9, or expressing a disruptive mutant make more massive stress fibers that are less dynamic than those in WT cells, leading to defects in cell motility and wound healing. The increase in stress fiber mass and stability can be explained, in part, by increased loading of the filament component tropomyosin onto stress fibers in the absence of α-actinin, as monitored via immunofluorescence. We show using imaging and cosedimentation that α-actinin and tropomyosin compete for binding to F-actin and that tropomyosin shields actin filaments from cofilin-mediated disassembly in vitro and in cells. Perturbing tropomyosin in cells lacking α-actinin-4 results in a complete loss of stress fibers. Our results with α-actinin-4 on stress fiber organization are the opposite of what might have been predicted from previous in vitro biochemistry and further highlight how the complex interactions of multiple proteins competing for filament binding lead to unexpected functions for actin-binding proteins in cells.
Keywords: actin; cofilin; contractility; epithelial cell; focal adhesions; kidney; migration; motility; paxillin; stress fibers; tropomyosin; α-actinin.
© 2018 Kemp and Brieher.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Isenberg G., Rathke P. C., Hülsmann N., Franke W. W., and Wohlfarth-Bottermann K. E. (1976) Cytoplasmic actomyosin fibrils in tissue culture cells: direct proof of contractility by visualization of ATP-induced contraction in fibrils isolated by laser micro-beam dissection. Cell Tissue Res. 166, 427–443 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

