Necroptosis in anti-viral inflammation
- PMID: 30050058
- PMCID: PMC6294789
- DOI: 10.1038/s41418-018-0172-x
Necroptosis in anti-viral inflammation
Abstract
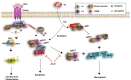
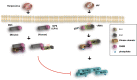
The primary function of the immune system is to protect the host from invading pathogens. In response, microbial pathogens have developed various strategies to evade detection and destruction by the immune system. This tug-of-war between the host and the pathogen is a powerful force that shapes organismal evolution. Regulated cell death (RCD) is a host response that limits the reservoir for intracellular pathogens such as viruses. Since pathogen-specific T cell and B cell responses typically take several days and is therefore slow-developing, RCD of infected cells during the first few days of the infection is critical for organismal survival. This innate immune response not only restricts viral replication, but also serves to promote anti-viral inflammation through cell death-associated release of damage-associated molecular patterns (DAMPs). In recent years, necroptosis has been recognized as an important response against many viruses. The central adaptor for necroptosis, RIPK3, also exerts anti-viral effects through cell death-independent activities such as promoting cytokine gene expression. Here, we will discuss recent advances on how viruses counteract this host defense mechanism and the effect of necroptosis on the anti-viral inflammatory reaction.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

