Efficacy and safety assessment of apatinib in patients with advanced gastric cancer: a meta-analysis
- PMID: 30050306
- PMCID: PMC6056163
- DOI: 10.2147/OTT.S157466
Efficacy and safety assessment of apatinib in patients with advanced gastric cancer: a meta-analysis
Abstract
Aim: This meta-analysis was performed to evaluate the efficacy and safety of apatinib in patients with advanced gastric cancer (AGC).
Methods: After evaluating the inclusion and exclusion criteria, the data of eligible randomized clinical trials (RCTs) were extracted. Outcomes including objective response rate (ORR), disease control rate (DCR), and adverse events (AEs) were analyzed in the meta-analysis.
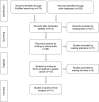
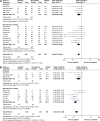
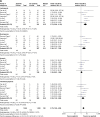
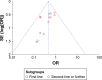
Results: Data of 1,069 patients from 13 RCTs were statistically analyzed. Pooled odds ratio (OR) for ORR and DCR was found to be 0.46 (95% confidence interval [CI]: 0.33, 0.64; P<0.00001) and 0.23 (95% CI: 0.15, 0.36; P<0.00001), respectively. Compared with placebo, apatinib showed statistical significance in AEs at any grade, including leucopenia, neutropenia, thrombocytopenia, diarrhea, hypertension, proteinuria, hand-foot syndrome, and fatigue (all P<0.05).
Conclusion: The results of our meta-analysis revealed that apatinib shows short-term efficacy over no-apatinib regimens or placebo regardless of its use as first- or second-line chemotherapy or for further treatment in patients with AGC accompanied with apparent AEs of any grade.
Keywords: adverse events; disease control rate; objective response rate.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Cunningham D, Allum WH, Stenning SP, et al. MGIC Trial Participants Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. ToGA Trial Investigators Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. - PubMed
-
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29(30):3968–3976. - PubMed
-
- Fuchs CS, Tomasek J, Yong CJ, et al. REGARD Trial Investigators Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

