Cladribine tablets' potential role as a key example of selective immune reconstitution therapy in multiple sclerosis
- PMID: 30050387
- PMCID: PMC6053904
- DOI: 10.2147/DNND.S161450
Cladribine tablets' potential role as a key example of selective immune reconstitution therapy in multiple sclerosis
Abstract
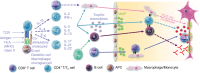
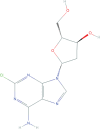
Multiple sclerosis (MS) is one of the most important, disabling, and prevalent neurological disorders of young adults. It is a chronic inflammatory and neurodegenerative disease when autoreactive B and T cells have downstream effects that result in demyelination and neuronal loss. Anti-inflammatory disease-modifying therapies do have proven efficacy in delaying disease and disability progression in MS. While the progress in MS treatments has already improved the prognosis and quality of patients' lives overall, there are some clear shortcomings and unmet needs in the current MS treatment landscape. The most promising means of MS treatment is selective immune reconstitution therapy (SIRT). This therapy is given in short-duration courses of immunosuppression, producing durable effects on the immune system and preventing nervous tissue loss. This review discusses the mechanisms of action and the data of clinical trials of cladribine tablets as an example of SIRT in MS. The clinical benefits of cladribine tablets in these studies include decreased relapse rate and disability progression with large reductions in lesion activity, and protection against brain volume loss. Whether all of these neurological findings are direct results of lymphocyte depletion, or if there are downstream effects on other, unknown, neurodegenerative processes are yet to be determined, but these clearly point to an interesting area of research.
Keywords: cladribine; multiple sclerosis; selective immune reconstitution; therapy.
Conflict of interest statement
Disclosure Boyko AN received honoraria as member of working groups, advisory boards, and participated in clinical trials supported by Biogen, Schering, Merck, Teva, Novartis, Sanofi-Genzyme, Actelion, Biocad, and Generium. Boyko OV participated in clinical trials supported by Biogen, Merck, Teva, Novartis, Sanofi-Genzyme, Biocad, and Generium.
Figures

References
-
- Multiple Sclerosis International Federation Atlas of MS 2013. Mapping multiple sclerosis around the world. 2013. Available from: https://www.msif.org/about-us/who-we-are-and-what-we-do/advocacy/atlas/.
-
- Cohen JA, Rae-Grant A. In: Handbook of Multiple Sclerosis. 2nd ed. Salazar T, editor. London: Springer Healthcare; 2012. pp. 1–6.
-
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

