The Axon-Myelin Unit in Development and Degenerative Disease
- PMID: 30050403
- PMCID: PMC6050401
- DOI: 10.3389/fnins.2018.00467
The Axon-Myelin Unit in Development and Degenerative Disease
Abstract
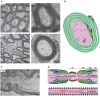
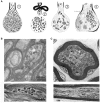
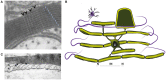
Axons are electrically excitable, cable-like neuronal processes that relay information between neurons within the nervous system and between neurons and peripheral target tissues. In the central and peripheral nervous systems, most axons over a critical diameter are enwrapped by myelin, which reduces internodal membrane capacitance and facilitates rapid conduction of electrical impulses. The spirally wrapped myelin sheath, which is an evolutionary specialisation of vertebrates, is produced by oligodendrocytes and Schwann cells; in most mammals myelination occurs during postnatal development and after axons have established connection with their targets. Myelin covers the vast majority of the axonal surface, influencing the axon's physical shape, the localisation of molecules on its membrane and the composition of the extracellular fluid (in the periaxonal space) that immerses it. Moreover, myelinating cells play a fundamental role in axonal support, at least in part by providing metabolic substrates to the underlying axon to fuel its energy requirements. The unique architecture of the myelinated axon, which is crucial to its function as a conduit over long distances, renders it particularly susceptible to injury and confers specific survival and maintenance requirements. In this review we will describe the normal morphology, ultrastructure and function of myelinated axons, and discuss how these change following disease, injury or experimental perturbation, with a particular focus on the role the myelinating cell plays in shaping and supporting the axon.
Keywords: Schwann cell; axonal transport; cytoskeleton; energy; morphology; neuroinflammation; oligodendrocyte.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

