Beneficial Effect of a Selective Adenosine A2A Receptor Antagonist in the APPswe/PS1dE9 Mouse Model of Alzheimer's Disease
- PMID: 30050407
- PMCID: PMC6052540
- DOI: 10.3389/fnmol.2018.00235
Beneficial Effect of a Selective Adenosine A2A Receptor Antagonist in the APPswe/PS1dE9 Mouse Model of Alzheimer's Disease
Abstract
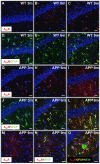
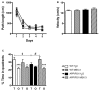
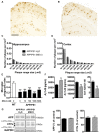
Consumption of caffeine, a non-selective adenosine A2A receptor (A2AR) antagonist, reduces the risk of developing Alzheimer's disease (AD) and mitigates both amyloid and Tau lesions in transgenic mouse models of the disease. While short-term treatment with A2AR antagonists have been shown to alleviate cognitive deficits in mouse models of amyloidogenesis, impact of a chronic and long-term treatment on the development of amyloid burden, associated neuroinflammation and memory deficits has never been assessed. In the present study, we have evaluated the effect of a 6-month treatment of APPsw/PS1dE9 mice with the potent and selective A2AR antagonist MSX-3 from 3 to 9-10 months of age. At completion of the treatment, we found that the MSX-3 treatment prevented the development of memory deficits in APP/PS1dE9 mice, without significantly altering hippocampal and cortical gene expressions. Interestingly, MSX-3 treatment led to a significant decrease of Aβ1-42 levels in the cortex of APP/PS1dE9 animals, while Aβ1-40 increased, thereby strongly affecting the Aβ1-42/Aβ1-40 ratio. Together, these data support the idea that A2AR blockade is of therapeutic value for AD.
Keywords: A2A; Alzheimer’s disease; adenosine receptor; amyloid; memory.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

