The Roles of Rods, Cones, and Melanopsin in Photoresponses of M4 Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs) and Optokinetic Visual Behavior
- PMID: 30050414
- PMCID: PMC6052130
- DOI: 10.3389/fncel.2018.00203
The Roles of Rods, Cones, and Melanopsin in Photoresponses of M4 Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs) and Optokinetic Visual Behavior
Abstract
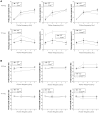
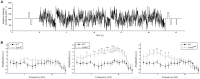
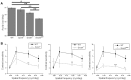
Intrinsically photosensitive retinal ganglion cells (ipRGCs) mediate not only image-forming vision like other ganglion cells, but also non-image-forming physiological responses to light such as pupil constriction and circadian photoentrainment. All ipRGCs respond to light through their endogenous photopigment melanopsin as well as rod/cone-driven synaptic inputs. A major knowledge gap is how melanopsin, rods, and cones differentially drive ipRGC photoresponses and image-forming vision. We whole-cell-recorded from M4-type ipRGCs lacking melanopsin, rod input, or cone input to dissect the roles of each component in ipRGCs' responses to steady and temporally modulated (≥0.3 Hz) lights. We also used a behavioral assay to determine how the elimination of melanopsin, rod, or cone function impacts the optokinetic visual behavior of mice. Results showed that the initial, transient peak in an M4 cell's responses to 10-s light steps arises from rod and cone inputs. Both the sustainability and poststimulus persistence of these light-step responses depend only on rod and/or cone inputs, which is unexpected because these ipRGC photoresponse properties have often been attributed primarily to melanopsin. For temporally varying stimuli, the enhancement of response sustainedness involves melanopsin, whereas stimulus tracking is mediated by rod and cone inputs. Finally, the behavioral assay showed that while all three photoreceptive systems are nearly equally important for contrast sensitivity, only cones and rods contribute to spatial acuity.
Keywords: cone; intrinsically photosensitive retinal ganglion cell (ipRGC); melanopsin; photoreceptor; retina; rod; vision; visual behavior.
Figures






References
-
- Berson D. M. (2008). Retinal ganglion cell types and their central projections. in The Senses: a Comprehensive Reference, Vol. 1, Vision I, eds Masland R. H., Albright T. (San Diego, CA: Academic Press; ), 491–519.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

