Pre-clinical Pharmacokinetic and Metabolomic Analyses of Isorhapontigenin, a Dietary Resveratrol Derivative
- PMID: 30050440
- PMCID: PMC6050476
- DOI: 10.3389/fphar.2018.00753
Pre-clinical Pharmacokinetic and Metabolomic Analyses of Isorhapontigenin, a Dietary Resveratrol Derivative
Abstract
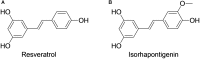
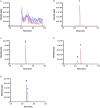
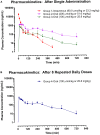
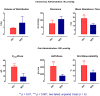
Background: Isorhapontigenin (trans-3,5,4'-trihydroxy-3'-methoxystilbene, ISO), a dietary resveratrol (trans-3,5,4'-trihydroxystilbene) derivative, possesses various health-promoting activities. To further evaluate its medicinal potentials, the pharmacokinetic and metabolomic profiles of ISO were examined in Sprague-Dawley rats. Methods: The plasma pharmacokinetics and metabolomics were monitored by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS), respectively. Results: Upon intravenous injection (90 μmol/kg), ISO exhibited a fairly rapid clearance (CL) and short mean residence time (MRT). After a single oral administration (100 μmol/kg), ISO was rapidly absorbed and showed a long residence in the systemic circulation. Dose escalation to 200 μmol/kg resulted in higher dose-normalized maximal plasma concentrations (Cmax/Dose), dose-normalized plasma exposures (AUC/Dose), and oral bioavailability (F). One-week repeated daily dosing of ISO did not alter its major oral pharmacokinetic parameters. Pharmacokinetic comparisons clearly indicated that ISO displayed pharmacokinetic profiles superior to resveratrol as its Cmax/Dose, AUC/Dose, and F were approximately two to three folds greater than resveratrol. Metabolomic investigation revealed that 1-week ISO administration significantly reduced plasma concentrations of arachidonic acid, cholesterol, fructose, allantoin, and cadaverine but increased tryptamine levels, indicating its impact on metabolic pathways related to health-promoting effects. Conclusion: ISO displayed favorable pharmacokinetic profiles and may be a promising nutraceutical in view of its health-promoting properties.
Keywords: isorhapontigenin; metabolomics; oral bioavailability; pharmacokinetics; resveratrol.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

