Novel Microscopic Techniques for Podocyte Research
- PMID: 30050501
- PMCID: PMC6050355
- DOI: 10.3389/fendo.2018.00379
Novel Microscopic Techniques for Podocyte Research
Abstract
Together with endothelial cells and the glomerular basement membrane, podocytes form the size-specific filtration barrier of the glomerulus with their interdigitating foot processes. Since glomerulopathies are associated with so-called foot process effacement-a severe change of well-formed foot processes into flat and broadened processes-visualization of the three-dimensional podocyte morphology is a crucial part for diagnosis of nephrotic diseases. However, interdigitating podocyte foot processes are too narrow to be resolved by classic light microscopy due to Ernst Abbe's law making electron microscopy necessary. Although three dimensional electron microscopy approaches like serial block face and focused ion beam scanning electron microscopy and electron tomography allow volumetric reconstruction of podocytes, these techniques are very time-consuming and too specialized for routine use or screening purposes. During the last few years, different super-resolution microscopic techniques were developed to overcome the optical resolution limit enabling new insights into podocyte morphology. Super-resolution microscopy approaches like three dimensional structured illumination microscopy (3D-SIM), stimulated emission depletion microscopy (STED) and localization microscopy [stochastic optical reconstruction microscopy (STORM), photoactivated localization microscopy (PALM)] reach resolutions down to 80-20 nm and can be used to image and further quantify podocyte foot process morphology. Furthermore, in vivo imaging of podocytes is essential to study the behavior of these cells in situ. Therefore, multiphoton laser microscopy was a breakthrough for in vivo studies of podocytes in transgenic animal models like rodents and zebrafish larvae because it allows imaging structures up to several hundred micrometer in depth within the tissue. Additionally, along with multiphoton microscopy, lightsheet microscopy is currently used to visualize larger tissue volumes and therefore image complete glomeruli in their native tissue context. Alongside plain visualization of cellular structures, atomic force microscopy has been used to study the change of mechanical properties of podocytes in diseased states which has been shown to be a culprit in podocyte maintenance. This review discusses recent advances in the field of microscopic imaging and demonstrates their currently used and other possible applications for podocyte research.
Keywords: STED microscopy; atomic force microscopy; light-sheet imaging; multiphoton imaging; podocyte nephropathy; serial block-face scanning electron microscopy (SBFSEM); structured illumination microscopy; superresolution microscopy.
Figures

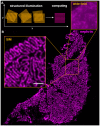
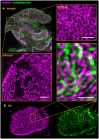
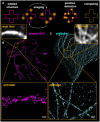
References
-
- Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. (1988) 59:673–82. - PubMed
-
- Bohman SO, Jaremko G, Bohlin AB, Berg U. Foot process fusion and glomerular filtration rate in minimal change nephrotic syndrome. Kidney Int. (1984) 25:696–700. - PubMed
-
- Abbe E. Beiträge zur theorie des mikroskops und der mikroskopischen wahrnehmung. Arch Mikrosk Anat. (1873) 9:418–40.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

