Rethinking Carbohydrate Synthesis: Stereoretentive Reactions of Anomeric Stannanes
- PMID: 30051523
- PMCID: PMC7265906
- DOI: 10.1002/chem.201803082
Rethinking Carbohydrate Synthesis: Stereoretentive Reactions of Anomeric Stannanes
Abstract
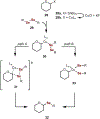
In this Concept article, recent advances are highlighted in the synthesis and applications of anomeric nucleophiles, a class of carbohydrates in which the C1 carbon bears a carbon-metal bond. First, the advantages of exploiting the carboanionic reactivity of carbohydrates and the methods for the synthesis of mono- and oligosaccharide stannanes are discussed. Second, recent developments in the glycosyl cross-coupling method resulting in the transfer of anomeric configuration from C1 stannanes to C-aryl glycosides are reviewed. These highly stereoretentive processes are ideally suited for the preparation of carbohydrate-based therapeutics and were demonstrated in the synthesis of antidiabetic drugs. Next, the application of the glycosyl cross-coupling method to the preparation of Se-glycosides and to glycodiversification of small molecules and peptides are highlighted. These reactions proceed with exclusive anomeric control for a broad range of substrates and tolerate carbohydrates with free hydroxyl groups. Taken together, anomeric nucleophiles have emerged as powerful tools for the synthesis of oligosaccharides and glycoconjugates and their future applications will open new possibilities to incorporate saccharides into small molecules and biologics.
Keywords: carbohydrates; copper; cross-coupling; palladium; synthetic methods.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures



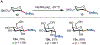

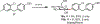



References
-
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, Essentials of Glycobiology, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2009; - PubMed
- Gabius H-J, The Sugar Code: Fundamentals of Glycosciences, Wiley-Blackwell, Hoboken, 2009.
-
- Nigudkar SS, Demchenko AV, Chem. Sci 2015, 6, 2687–2704; - PMC - PubMed
- Bennett CS, Selective Glycosylations: Synthetic Methods and Catalysts: Synthetic Methods and Catalysts, Wiley, Hoboken, 2017;
- Kulkarni SS, Wang C-C, Sabbavarapu NM, Podilapu AR, Liao P-H, Hung S-C, Chem. Rev 2018, 118, 8025–8104. - PubMed
-
- Frihed TG, Bols M, Pedersen CM, Chem. Rev 2015, 115, 4963–5013. - PubMed
-
- Zhu X, Schmidt RR, Angew. Chem. Int. Ed 2009, 48, 1900; - PubMed
- Angew. Chem 2009, 121, 1932.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

