Increased transport of acetyl-CoA into the endoplasmic reticulum causes a progeria-like phenotype
- PMID: 30051577
- PMCID: PMC6156544
- DOI: 10.1111/acel.12820
Increased transport of acetyl-CoA into the endoplasmic reticulum causes a progeria-like phenotype
Abstract
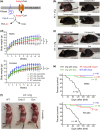
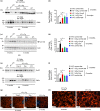
The membrane transporter AT-1/SLC33A1 translocates cytosolic acetyl-CoA into the lumen of the endoplasmic reticulum (ER), participating in quality control mechanisms within the secretory pathway. Mutations and duplication events in AT-1/SLC33A1 are highly pleiotropic and have been linked to diseases such as spastic paraplegia, developmental delay, autism spectrum disorder, intellectual disability, propensity to seizures, and dysmorphism. Despite these known associations, the biology of this key transporter is only beginning to be uncovered. Here, we show that systemic overexpression of AT-1 in the mouse leads to a segmental form of progeria with dysmorphism and metabolic alterations. The phenotype includes delayed growth, short lifespan, alopecia, skin lesions, rectal prolapse, osteoporosis, cardiomegaly, muscle atrophy, reduced fertility, and anemia. In terms of homeostasis, the AT-1 overexpressing mouse displays hypocholesterolemia, altered glycemia, and increased indices of systemic inflammation. Mechanistically, the phenotype is caused by a block in Atg9a-Fam134b-LC3β and Atg9a-Sec62-LC3β interactions, and defective reticulophagy, the autophagic recycling of the ER. Inhibition of ATase1/ATase2 acetyltransferase enzymes downstream of AT-1 restores reticulophagy and rescues the phenotype of the animals. These data suggest that inappropriately elevated acetyl-CoA flux into the ER directly induces defects in autophagy and recycling of subcellular structures and that this diversion of acetyl-CoA from cytosol to ER is causal in the progeria phenotype. Collectively, these data establish the cytosol-to-ER flux of acetyl-CoA as a novel event that dictates the pace of aging phenotypes and identify intracellular acetyl-CoA-dependent homeostatic mechanisms linked to metabolism and inflammation.
Keywords: AT-1/SLC33A1; ATase1; ATase2; acetyl-CoA; lysine acetylation; progeria.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures





References
-
- Ding, Y. , Dellisanti, C. D. , Ko, M. H. , Czajkowski, C. , & Puglielli, L. (2014). The endoplasmic reticulum‐based acetyltransferases, ATase1 and ATase2, associate with the oligosaccharyl‐transferase to acetylate correctly folded polypeptides. Journal of Biological Chemistry, 289(46), 32044–32055. 10.1074/jbc.M114.585547 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- T32 HL007936/HL/NHLBI NIH HHS/United States
- R01 DK050107/DK/NIDDK NIH HHS/United States
- R01 DK102948/DK/NIDDK NIH HHS/United States
- R21 AG051974/AG/NIA NIH HHS/United States
- K01 DK113117/DK/NIDDK NIH HHS/United States
- R01 DK108259/DK/NIDDK NIH HHS/United States
- R01 NS094154/NS/NINDS NIH HHS/United States
- K99 AG041765/AG/NIA NIH HHS/United States
- U54 HD090256/HD/NICHD NIH HHS/United States
- R01 CA152108/CA/NCI NIH HHS/United States
- R01 HL113066/HL/NHLBI NIH HHS/United States
- R00 AG041765/AG/NIA NIH HHS/United States
- RF1 AG053937/AG/NIA NIH HHS/United States
- T32 AG000213/AG/NIA NIH HHS/United States
- P50 AG033514/AG/NIA NIH HHS/United States
- R37 DK050107/DK/NIDDK NIH HHS/United States
- RF1 AG057408/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

