Prognosis of patients with intermediate risk IPSS-R myelodysplastic syndrome indicates variable outcomes and need for models beyond IPSS-R
- PMID: 30051599
- PMCID: PMC6750209
- DOI: 10.1002/ajh.25234
Prognosis of patients with intermediate risk IPSS-R myelodysplastic syndrome indicates variable outcomes and need for models beyond IPSS-R
Abstract
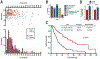
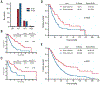
The International Prognostic Scoring System-Revised (IPSS-R) is one standard for myelodysplastic syndrome (MDS) risk stratification. It divides patients into five categories including an intermediate subset (IPSS-R int-risk). Outcomes and clinical interventions for patients with IPSS-R int-risk are not well defined. We performed an analysis of outcomes of this group of patients. Out of 3167 patients, a total of 298 were identified with IPSS-R int-risk MDS and retrospectively analyzed to assess characteristics affecting outcomes. Cox proportional hazard models for overall survival (OS) were performed to identify statistically significant clinical factors that influence survival. Age of 66 years or greater, peripheral blood blasts of 2% or more, and history of red blood cell (RBC) transfusion were significantly associated with inferior survival. Based on these features, MDS patients with IPSS-R int-risk were classified into two prognostic risk groups for analysis, an int-favorable group and an int-adverse group, and had significantly divergent outcomes. Sequential prognostication was validated using two independent datasets comprising over 700 IPSS-R int-risk patients. The difference in median survival between int-favorable and int-adverse patients was 3.7 years in the test cohort, and 1.8 and 2.0 years in the two validation cohorts. These results confirm significantly variable outcomes of patients with IPSS-R int-risk and need for different prognostic systems.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of Interest Disclosures
The authors have no conflicts to report.
Figures
References
-
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. - PubMed
-
- Kouides PA, Bennett JM. Understanding the Myelodysplastic Syndromes. Oncologist. 1997;2(6):389–401. - PubMed
-
- Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network. 2017;15(1):60–87. - PubMed
-
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
