Cascading speciation among mutualists and antagonists in a tree-beetle-fungi interaction
- PMID: 30051849
- PMCID: PMC6030525
- DOI: 10.1098/rspb.2018.0694
Cascading speciation among mutualists and antagonists in a tree-beetle-fungi interaction
Abstract
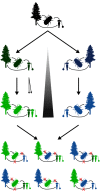
Cascading speciation is predicted to occur when multiple interacting species diverge in parallel as a result of divergence in one species promoting adaptive differentiation in other species. However, there are few examples where ecological interactions among taxa have been shown to result in speciation that cascades across multiple trophic levels. Here, we test for cascading speciation occurring among the western pine beetle (Dendroctonus brevicomis), its primary host tree (Pinus ponderosa), and the beetle's fungal mutualists (Ceratocystiopsis brevicomi and Entomocorticium sp. B). We assembled genomes for the beetle and a fungal symbiont and then generated reduced representation genomic data (RADseq) from range-wide samples of these three interacting species. Combined with published data for the host tree, we present clear evidence that the tree, the beetle, and the fungal symbionts are all genetically structured into at least two distinct groups that have strongly codiverged with geographical isolation. We then combine our genomic results with diverse population and laboratory-based data to show evidence for reproductive isolation at each level of the cascade and for coevolution of both antagonistic and mutualistic species interactions within this complex network.
Keywords: coevolution; diversification; ectosymbiosis; mutualism; symbiosis.
© 2018 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates.
-
- Forister ML, Feldman CR. 2011. Phylogenetic cascades and the origins of tropical diversity. Biotropica 43, 270–278. ( 10.1111/j.1744-7429.2010.00702.x) - DOI
-
- Abrahamson WG, Blair CP. 2008. Sequential radiation through host-race formation: herbivore diversity leads to diversity in natural enemies. In Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects (ed. Tilmon KJ.), pp. 182–202. Berkeley, CA: University of California Press.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
