Enthalpic stabilization of an SH3 domain by D2 O
- PMID: 30052291
- PMCID: PMC6194290
- DOI: 10.1002/pro.3477
Enthalpic stabilization of an SH3 domain by D2 O
Abstract
The stability of a protein is vital for its biological function, and proper folding is partially driven by intermolecular interactions between protein and water. In many studies, H2 O is replaced by D2 O because H2 O interferes with the protein signal. Even this small perturbation, however, affects protein stability. Studies in isotopic waters also might provide insight into the role of solvation and hydrogen bonding in protein folding. Here, we report a complete thermodynamic analysis of the reversible, two-state, thermal unfolding of the metastable, 7-kDa N-terminal src-homology 3 domain of the Drosophila signal transduction protein drk in H2 O and D2 O using one-dimensional 19 F NMR spectroscopy. The stabilizing effect of D2 O compared with H2 O is enthalpic and has a small to insignificant effect on the temperature of maximum stability, the entropy, and the heat capacity of unfolding. We also provide a concise summary of the literature about the effects of D2 O on protein stability and integrate our results into this body of data.
Keywords: NMR spectroscopy; SH3 domain; deuterium oxide; protein folding; protein stability; solvent isotope effect; thermodynamics.
© 2018 The Protein Society.
Figures
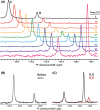
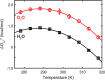
References
-
- Chaplin MF (2001) Water: its importance to life. Biochem Mol Bio Edu 29:54–59.
-
- Chaplin M (2006) Do we underestimate the importance of water in cell biology? Nat Rev Mol Cell Bio 7:861–866. - PubMed
-
- Fujita Y, Noda Y (1981) Effect of hydration on the thermal stability of protein as measured by differential scanning calorimetry. Chymotrypsinogen A. Int J Pept Protein Res 18:12–17. - PubMed
-
- Privalov PL, Makhatadze GI (1993) Contribution of hydration to protein folding thermodynamics. II. The entropy and Gibbs energy of hydration. J Mol Biol 232:660–679. - PubMed
-
- Makhatadze GI, Privalov PL (1993) Contribution of hydration to protein folding thermodynamics. I. The enthalpy of hydration. J Mol Biol 232:639–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

