Response of Arabidopsis primary metabolism and circadian clock to low night temperature in a natural light environment
- PMID: 30053131
- PMCID: PMC6137998
- DOI: 10.1093/jxb/ery276
Response of Arabidopsis primary metabolism and circadian clock to low night temperature in a natural light environment
Abstract
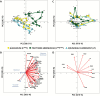
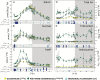
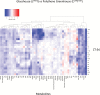
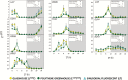
Plants are exposed to varying irradiance and temperature within a day and from day to day. We previously investigated metabolism in a temperature-controlled greenhouse at the spring equinox on both a cloudy and a sunny day [daily light integral (DLI) of 7 mol m-2 d-1 and 12 mol m-2 d-1]. Diel metabolite profiles were largely captured in sinusoidal simulations at similar DLIs in controlled-environment chambers, except that amino acids were lower in natural light regimes. We now extend the DLI12 study by investigating metabolism in a natural light regime with variable temperature including cool nights. Starch was not completely turned over, anthocyanins and proline accumulated, and protein content rose. Instead of decreasing, amino acid content rose. Connectivity in central metabolism, which decreased in variable light, was not further weakened by variable temperature. We propose that diel metabolism operates better when light and temperature are co-varying. We also compared transcript abundance of 10 circadian clock genes in this temperature-variable regime with the temperature-controlled natural and sinusoidal light regimes. Despite temperature compensation, peak timing and abundance for dawn- and day-phased genes and GIGANTEA were slightly modified in the variable temperature treatment. This may delay dawn clock activity until the temperature rises enough to support rapid metabolism and photosynthesis.
Figures


Comment in
-
Growing plants in fluctuating environments: why bother?J Exp Bot. 2018 Sep 14;69(20):4651-4654. doi: 10.1093/jxb/ery312. J Exp Bot. 2018. PMID: 30307518 Free PMC article.
References
-
- Apelt F, Breuer D, Nikoloski Z, Stitt M, Kragler F. 2015. Phytotyping4D: a light-field imaging system for non-invasive and accurate monitoring of spatio-temporal plant growth. The Plant Journal 82, 693–706. - PubMed
-
- Atkin OK, Tjoelker MG. 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8, 343–351. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

