How Macrolide Antibiotics Work
- PMID: 30054232
- PMCID: PMC6108949
- DOI: 10.1016/j.tibs.2018.06.011
How Macrolide Antibiotics Work
Abstract
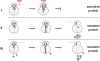
Macrolide antibiotics inhibit protein synthesis by targeting the bacterial ribosome. They bind at the nascent peptide exit tunnel and partially occlude it. Thus, macrolides have been viewed as 'tunnel plugs' that stop the synthesis of every protein. More recent evidence, however, demonstrates that macrolides selectively inhibit the translation of a subset of cellular proteins, and that their action crucially depends on the nascent protein sequence and on the antibiotic structure. Therefore, macrolides emerge as modulators of translation rather than as global inhibitors of protein synthesis. The context-specific action of macrolides is the basis for regulating the expression of resistance genes. Understanding the details of the mechanism of macrolide action may inform rational design of new drugs and unveil important principles of translation regulation.
Keywords: antibiotic; ketolide; macrolide; resistance; ribosome; translation.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


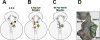


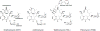

References
-
- Wilson DN. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol. 2014;12:35–48. - PubMed
-
- Contreras A, Vazquez D. Cooperative and antagonistic interactions of peptidyl-tRNA and antibiotics with bacterial ribosomes. Eur J Biochem. 1977;74:539–547. - PubMed
-
- Odom OW, et al. The synthesis of polyphenylalanine on ribosomes to which erythromycin is bound. Eur J Biochem. 1991;198:713–722. - PubMed
-
- Arevalo MA, et al. Protein components of the erythromycin binding site in bacterial ribosomes. J Biol Chem. 1988;263:58–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

