Amelioration of Diabetic Nephropathy Using a Retinoic Acid Receptor β 2 Agonist
- PMID: 30054312
- PMCID: PMC6123666
- DOI: 10.1124/jpet.118.249375
Amelioration of Diabetic Nephropathy Using a Retinoic Acid Receptor β 2 Agonist
Abstract
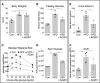
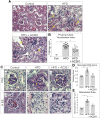
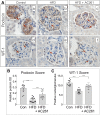
Vitamin A (VA) and its derivatives, known as retinoids, play critical roles in renal development through retinoic acid receptor β2 (RARβ2). Disruptions in VA signaling pathways are associated with the onset of diabetic nephropathy (DN). Despite the known role of RARβ2 in renal development, the effects of selective agonists for RARβ2 in a high-fat diet (HFD) model of DN are unknown. Here we examined whether AC261066 (AC261), a highly selective agonist for RARβ2, exhibited therapeutic effects in a HFD model of DN in C57BL/6 mice. Twelve weeks of AC261 administration to HFD-fed mice was well tolerated with no observable side effects. Compared with HFD-fed mice, HFD + AC261-treated mice had improved glycemic control and reductions in proteinuria and urine albumin-to-creatinine ratio. Several cellular hallmarks of DN were mitigated in HFD + AC261-treated mice, including reductions in tubule lipid droplets, podocyte (POD) effacement, endothelial cell collapse, mesangial expansion, and glomerular basement membrane thickening. Mesangial and tubule interstitial expression of the myofibroblast markers α-smooth muscle actin (α-SMA) and type IV collagen (Col-IV) was lower in HFD + AC261-treated mice compared with HFD alone. Ultrastructural and immunohistochemistry analyses showed that, compared with HFD-fed mice, HFD + AC261-treated mice showed preservation of POD foot process and slit-diaphragm morphology, an increase in the levels of slit-diagram protein podocin, and the transcription factor Wilms tumor-suppressor gene 1 in PODs. Given the need for novel DN therapies, our results warrant further studies of the therapeutic properties of AC261 in DN.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Anderson RJ, Ray CJ, Hattler BG. (1998) Retinoic acid regulation of renal tubular epithelial and vascular smooth muscle cell function. J Am Soc Nephrol 9:773–781. - PubMed
-
- Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, Schuchardt A, Bacallao RL, Mendelsohn CL. (2002) Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet 32:109–115. - PubMed
-
- Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. (2001) Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27:74–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

