High-throughput determination of RNA structures
- PMID: 30054568
- PMCID: PMC7388734
- DOI: 10.1038/s41576-018-0034-x
High-throughput determination of RNA structures
Abstract
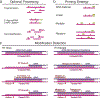
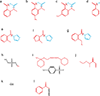
RNA performs and regulates a diverse range of cellular processes, with new functional roles being uncovered at a rapid pace. Interest is growing in how these functions are linked to RNA structures that form in the complex cellular environment. A growing suite of technologies that use advances in RNA structural probes, high-throughput sequencing and new computational approaches to interrogate RNA structure at unprecedented throughput are beginning to provide insights into RNA structures at new spatial, temporal and cellular scales.
Conflict of interest statement
Competing interests
The authors declare no competing financial interests.
Figures


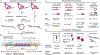

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

