Using the Influenza Patient-reported Outcome (FLU-PRO) diary to evaluate symptoms of influenza viral infection in a healthy human challenge model
- PMID: 30055573
- PMCID: PMC6064178
- DOI: 10.1186/s12879-018-3220-8
Using the Influenza Patient-reported Outcome (FLU-PRO) diary to evaluate symptoms of influenza viral infection in a healthy human challenge model
Abstract
Background: In clinical studies involving a healthy volunteer human challenge model, a valid and reliable measure to assess the evolution of patient-reported symptom type and severity following viral exposure is necessary. This study examines the use of the InFLUenza Patient-Reported Outcome (FLU-PRO) diary as a standardized measure of symptom severity in a healthy volunteer human challenge model.
Methods: Healthy adults admitted to the NIH Clinical Center (Day - 1) underwent a 9-day inpatient quarantine after intranasal challenge with a wild-type influenza A/H1N1pdm virus (Day 0). Participants completed the 32-item FLU-PRO diary twice daily for 14 days to assess presence, severity, and duration of symptoms across six body systems. Secondary analyses included descriptive statistics to examine FLU-PRO scores over the course of illness and analysis of variance to compare scores on Day 3 post-challenge by presence of viral shedding, and pre-challenge hemagglutinin and neuraminidase inhibition (HAI and NAI) titers.
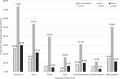
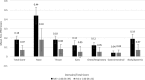
Results: All but one subject (99%), who was lost to follow-up, completed twice daily FLU-PRO diaries on all study assessment days. FLU-PRO demonstrated that 61 of 65 subjects reported symptoms (Days: Median 5, Mean 6 ± 7), of whom 37 (61%) had viral shedding. Pre-challenge, 39 (64%) and 10 (16%) subjects had low (< 1:40) HAI and NAI titers, respectively. Nose, throat, body, and gastrointestinal (GI) symptoms reached peak intensity at Day 3, followed by chest/respiratory and eye symptoms at Day 4. Subjects with viral shedding had higher mean FLU-PRO scores compared to those without, except for Eye and GI domains (p <0.05). Mean FLU-PRO scores were significantly higher for subjects with low NAI titer (p <0.05) across all domains. No significant differences were observed between HAI titer groups. FLU-PRO scores of the low HAI-low NAI group (n = 10) were significantly higher (more severe) than the other two groups (p < 0.05) (high HAI-high NAI (n = 22), low HAI-high NAI (n = 29)).
Conclusions: The FLU-PRO had high adherence and low respondent burden. It can be used to track symptom onset, intensity, duration, and recovery from influenza infection in clinical research. In this human challenge study, scores were responsive to change and distinguished known clinical subgroups.
Trial registration: NCT01971255 First Registered October 2, 2013.
Keywords: Human challenge study; Influenza; Symptoms; Virus.
Conflict of interest statement
Ethics approval and consent to participate
This study (
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- World Health Organization. Media centre. Influenza (Seasonal). Fact Sheet. 2016. Available at: http://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal). Accessed June 20, 2017.
-
- Food and Drug Administration Guidance for industry on influenza: developing drugs for treatment and/or prophylaxis. Fed Regist. 2011;76(71):20689–20690.
-
- Food and Drug Administration Guidance for industry on patient-reported outcome measures: use in medical product development to support labeling claims. Fed Regist. 2009;74(235):65132–65133.
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

