Signal Percolation within a Bacterial Community
- PMID: 30056004
- PMCID: PMC6214369
- DOI: 10.1016/j.cels.2018.06.005
Signal Percolation within a Bacterial Community
Abstract
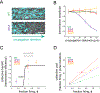
Signal transmission among cells enables long-range coordination in biological systems. However, the scarcity of quantitative measurements hinders the development of theories that relate signal propagation to cellular heterogeneity and spatial organization. We address this problem in a bacterial community that employs electrochemical cell-to-cell communication. We developed a model based on percolation theory, which describes how signals propagate through a heterogeneous medium. Our model predicts that signal transmission becomes possible when the community is organized near a critical phase transition between a disconnected and a fully connected conduit of signaling cells. By measuring population-level signal transmission with single-cell resolution in wild-type and genetically modified communities, we confirm that the spatial distribution of signaling cells is organized at the predicted phase transition. Our findings suggest that at this critical point, the population-level benefit of signal transmission outweighs the single-cell level cost. The bacterial community thus appears to be organized according to a theoretically predicted spatial heterogeneity that promotes efficient signal transmission.
Keywords: biofilms; criticality; percolation; self-organization; signal transmission.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Aharony A (1980). Universal critical amplitude ratios for percolation. Phys. Rev. B 22, 400–414.
-
- Alonso S, and Bär M (2016). Reentry produced by small-scale heterogeneities in a discrete model of cardiac tissue. J. Phys.: Conf. Ser. 727, 012002.
-
- Bak P, Chen K, and Tang C (1990). A forest-fire model and some thoughts on turbulence. Phys. Lett. A 147, 297–300.
-
- Bär M, Bangia AK, Kevrekidis IG, Haas G, Rotermund HH, and Ertl G (1996). Composite Catalyst Surfaces: Effect of Inert and Active Heterogeneities on Pattern Formation. J. Phys. Chem. 100, 19106–19117.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

