T rial of feasibility and acceptability of routine low-dose aspirin versus E arly S creening T est indicated aspirin for pre-eclampsia prevention (TEST study): a multicentre randomised controlled trial
- PMID: 30056389
- PMCID: PMC6067363
- DOI: 10.1136/bmjopen-2018-022056
T rial of feasibility and acceptability of routine low-dose aspirin versus E arly S creening T est indicated aspirin for pre-eclampsia prevention (TEST study): a multicentre randomised controlled trial
Abstract
Objective: Evaluate the feasibility and acceptability of routine aspirin in low-risk women, compared with screening-test indicated aspirin for the prevention of pre-eclampsia and fetal growth restriction.
Design: Multicentre open-label feasibility randomised controlled trial.
Setting: Two tertiary maternity hospitals in Dublin, Ireland.
Participants: 546 low-risk nulliparous women completed the study.
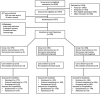
Interventions: Women underwent computerised randomisation to: Group 1-routine aspirin 75 mg from 11 until 36 weeks; Group 2-no aspirin and; Group 3-aspirin based on the Fetal Medicine Foundation screening test. PRIMARY AND SECONDARY OUTCOME MEASURES: (1) Proportion agreeing to participate; (2) compliance with protocol; (3) proportion where first trimester uterine artery Doppler was obtainable and; (4) time taken to issue a screening result. Secondary outcomes included rates of pre-eclampsia and small-for-gestational-age fetuses.
Results: 546 were included in the routine aspirin (n=179), no aspirin (n=183) and screen and treat (n=184) groups. 546 of 1054 were approached (51.8%) and enrolled. Average aspirin adherence was 90%. The uterine artery Doppler was obtained in 98.4% (181/184) and the average time to obtain a screening result was 7.6 (0-26) days. Of those taking aspirin, vaginal spotting was greater; n=29 (15.1%), non-aspirin n=28 (7.9%), OR 2.1 (95% CI 1.2 to 3.6). Postpartum haemorrhage >500 mL was also greater; aspirin n=26 (13.5%), no aspirin n=20 (5.6%), OR 2.6 (95% CI 1.4 to 4.8).
Conclusion: Low-risk nulliparous women are open to taking aspirin in pregnancy and had high levels of adherence. Aspirin use was associated with greater rates of vaginal bleeding. An appropriately powered randomised controlled trial is now required to address the efficacy and safety of universal low-dose aspirin in low-risk pregnancy compared with a screening approach.
Trial registration number: ISRCTN (15191778); Post-results.
Keywords: aspirin; feasibility; low risk; preeclampsia; screening.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Sibai BM, Caritis SN, Thom E, et al. . Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 1993;329:1213–8. 10.1056/NEJM199310213291701 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical