Forebrain Control of Behaviorally Driven Social Orienting in Zebrafish
- PMID: 30057306
- PMCID: PMC6082377
- DOI: 10.1016/j.cub.2018.06.016
Forebrain Control of Behaviorally Driven Social Orienting in Zebrafish
Abstract
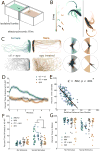
Deficits in social engagement are diagnostic of multiple neurodevelopmental disorders, including autism and schizophrenia [1]. Genetically tractable animal models like zebrafish (Danio rerio) could provide valuable insight into developmental factors underlying these social impairments, but this approach is predicated on the ability to accurately and reliably quantify subtle behavioral changes. Similarly, characterizing local molecular and morphological phenotypes requires knowledge of the neuroanatomical correlates of social behavior. We leveraged behavioral and genetic tools in zebrafish to both refine our understanding of social behavior and identify brain regions important for driving it. We characterized visual social interactions between pairs of adult zebrafish and discovered that they perform a stereotyped orienting behavior that reflects social attention [2]. Furthermore, in pairs of fish, the orienting behavior of one individual is the primary factor driving the same behavior in the other individual. We used manual and genetic lesions to investigate the forebrain contribution to this behavior and identified a population of neurons in the ventral telencephalon whose ablation suppresses social interactions, while sparing other locomotor and visual behaviors. These neurons are cholinergic and express the gene encoding the transcription factor Lhx8a, which is required for development of cholinergic neurons in the mouse forebrain [3]. The neuronal population identified in zebrafish lies in a region homologous to mammalian forebrain regions implicated in social behavior such as the lateral septum [4]. Our data suggest that an evolutionarily conserved population of neurons controls social orienting in zebrafish.
Keywords: basal telencephalon; collective behavior; lateral septum; reciprocal.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Social Behavior: A Neural Circuit for Social Behavior in Zebrafish.Curr Biol. 2018 Aug 6;28(15):R828-R830. doi: 10.1016/j.cub.2018.06.065. Curr Biol. 2018. PMID: 30086314
References
-
- Fakhoury M. Autism spectrum disorders: A review of clinical features, theories, and diagnosis. Int J Dev Neurosci. 2015;43:70–7. - PubMed
-
- Clarke A, File SE. Selective neurotoxin lesions of the lateral septum: changes in social and aggressive behaviors. Pharmacol Biochem Behav. 1982;17(4):623–8. - PubMed
-
- Suriyampola PS, Shelton DS, Shukla R, Roy T, Bhat A, Martins EP. Zebrafish social behavior in the wild. Zebrafish. 2016;13(1):1–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

