Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy
- PMID: 30057551
- PMCID: PMC6053590
- DOI: 10.3389/fphys.2018.00820
Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy
Abstract
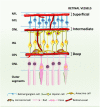
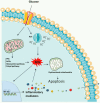
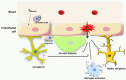
Oxidative stress plays key roles in the pathogenesis of retinal diseases, such as diabetic retinopathy. Reactive oxygen species (ROS) are increased in the retina in diabetes and the antioxidant defense system is also compromised. Increased ROS stimulate the release of pro-inflammatory cytokines, promoting a chronic low-grade inflammation involving various signaling pathways. An excessive production of ROS can lead to retinal endothelial cell injury, increased microvascular permeability, and recruitment of inflammatory cells at the site of inflammation. Recent studies have started unraveling the complex crosstalk between retinal endothelial cells and neuroglial cells or leukocytes, via both cell-to-cell contact and secretion of cytokines. This crosstalk is essential for the maintenance of the integrity of retinal vascular structure. Under diabetic conditions, an aberrant interaction between endothelial cells and other resident cells of the retina or invading inflammatory cells takes place in the retina. Impairment in the secretion and flow of molecular signals between different cells can compromise the retinal vascular architecture and trigger angiogenesis. In this review, the synergistic contributions of redox-inflammatory processes for endothelial dysfunction in diabetic retinopathy will be examined, with particular attention paid to endothelial cell communication with other retinal cells.
Keywords: apoptosis; blood–retinal barrier; diabetic retinopathy; inflammation; neovascularization; oxidative stress; retinal endothelial cells.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

