Purification and Characterisation of Malate Dehydrogenase From Synechocystis sp. PCC 6803: Biochemical Barrier of the Oxidative Tricarboxylic Acid Cycle
- PMID: 30057585
- PMCID: PMC6053527
- DOI: 10.3389/fpls.2018.00947
Purification and Characterisation of Malate Dehydrogenase From Synechocystis sp. PCC 6803: Biochemical Barrier of the Oxidative Tricarboxylic Acid Cycle
Erratum in
-
Corrigendum: Purification and Characterisation of Malate Dehydrogenase From Synechocystis sp. PCC 6803: Biochemical Barrier of the Oxidative Tricarboxylic Acid Cycle.Front Plant Sci. 2021 Jun 11;12:699371. doi: 10.3389/fpls.2021.699371. eCollection 2021. Front Plant Sci. 2021. PMID: 34178009 Free PMC article.
Abstract
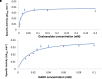
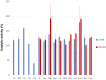
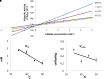
Cyanobacteria possess an atypical tricarboxylic acid (TCA) cycle with various bypasses. Previous studies have suggested that a cyclic flow through the TCA cycle is not essential for cyanobacteria under normal growth conditions. The cyanobacterial TCA cycle is, thus, different from that in other bacteria, and the biochemical properties of enzymes in this TCA cycle are less understood. In this study, we reveal the biochemical characteristics of malate dehydrogenase (MDH) from Synechocystis sp. PCC 6803 MDH (SyMDH). The optimal temperature of SyMDH activity was 45-50°C and SyMDH was more thermostable than MDHs from other mesophilic microorganisms. The optimal pH of SyMDH varied with the direction of the reaction: pH 8.0 for the oxidative reaction and pH 6.5 for the reductive reaction. The reductive reaction catalysed by SyMDH was activated by magnesium ions and fumarate, indicating that SyMDH is regulated by a positive feedback mechanism. The Km-value of SyMDH for malate was approximately 210-fold higher than that for oxaloacetate and the Km-value for NAD+ was approximately 19-fold higher than that for NADH. The catalytic efficiency of SyMDH for the reductive reaction, deduced from kcat-values, was also higher than that for the oxidative reaction. These results indicate that SyMDH is more efficient in the reductive reaction in the TCA cycle, and it plays key roles in determining the direction of the TCA cycle in this cyanobacterium.
Keywords: TCA cycle; biochemistry; cyanobacteria; malate dehydrogenase; metabolic enzyme.
Figures




References
-
- Broddrick J. T., Rubin B. E., Welkie D. G., Du N., Mih N., Diamond S., et al. (2016). Unique attributes of cyanobacterial metabolism revealed by improved genome-scale metabolic modeling and essential gene analysis. Proc. Natl. Acad. Sci. U.S.A. 113 E8344–E8353. 10.1073/pnas.1613446113 - DOI - PMC - PubMed
-
- Cendrin F., Chroboczek J., Zaccai G., Eisenberg H., Mevarech M. (1997). Cloning, sequencing, and expression in Escherichia coli of the gene coding for malate dehydrogenase of the extremely halophilic archaebacterium Haloarcula marismortui. Biochemistry 32 4308–4313. 10.1021/bi00067a020 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

