Gene therapy for the treatment of X-linked retinitis pigmentosa
- PMID: 30057863
- PMCID: PMC6059358
- DOI: 10.1080/21678707.2018.1444476
Gene therapy for the treatment of X-linked retinitis pigmentosa
Abstract
Introduction: X-linked retinitis pigmentosa caused by mutations in the retinitis pigmentosa GTPase regulator (RPGR) gene is the most common form of recessive RP. The phenotype is characterised by its severity and rapid disease progression. Gene therapy using adeno-associated viral vectors is currently the most promising therapeutic approach. However, the construction of a stable vector encoding the full-length RPGR transcript has previously proven to be a limiting step towards gene therapy clinical trials. Recently however, a codon optimised version of RPGR has been shown to increase the stability and fidelity of the sequence, conferring a therapeutic effect in murine and canine animal models.
Areas covered: This manuscript reviews the natural history of X-linked retinitis pigmentosa and the research performed from the discovery of the causative gene, RPGR, to the preclinical testing of potential therapies that have led to the initiation of three clinical trials.
Expert opinion: X-linked retinitis pigmentosa is an amenable disease to be treated by gene therapy. Codon optimisation has overcome the challenge of designing an RPGR vector without mutations, and with a therapeutic effect in different animal models. With the RPGR gene therapy clinical trials still in the early stages, the confirmation of the safety, tolerability and potency of the therapy is still ongoing.
Keywords: Adeno-associated virus; codon optimisation; gene therapy; retinitis pigmentosa; retinitis pigmentosa GTPase regulator (RPGR).
Conflict of interest statement
Declaration of Interest RE MacLaren is the academic founder and director of Nightstar Therapeutics, a gene therapy company established by the University of Oxford and originally funded by the Wellcome Trust through Syncona Partners. RE MacLaren and M. Dominik Fischer are names inventors on a patent filed on behalf of the University of Oxford, relating to the expression cassette and codon optimisation of RPGR coding sequence in general. They are also consultants to Nightstar Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Figures
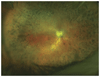
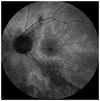


References
-
- Anasagasti A, Irigoyen C, Barandika O, et al. Current mutation discovery approaches in Retinitis Pigmentosa. Vision Res. 2012;75:117–129. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
